Article Text
Abstract
With significant advances in the management of gastrointestinal disease there has been a move from diagnosing advanced pathology, to detecting early lesions that are potentially amenable to curative endoscopic treatment. This has required an improvement in diagnostics, with a focus on identifying and characterising subtle mucosal changes. There is great interest in the use of optical technologies to predict histology and enable the formulation of a real-time in vivo diagnosis, a so-called ‘optical biopsy’. The aim of this review is to explore the evidence for the use of the current commercially available imaging techniques in the gastrointestinal tract.
- BARRETT'S OESOPHAGUS
- COLONIC ADENOMAS
- ENDOSCOPY
- GASTRIC NEOPLASIA
- GASTROINTESINAL ENDOSCOPY
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
The purpose of gastrointestinal endoscopy, which has evolved towards identifying early pathology and precursor lesions, has necessitated an improvement in technology. This has resulted in an array of new imaging systems, not all of which are widely available, as in most cases a significant financial investment in equipment needs to be made. As yet there is no single imaging modality that is applicable in all clinical scenarios, therefore an understanding of all of these techniques is required.
Currently available technology
High definition white light endoscopy
The standard of white light endoscopy (WLE) has improved dramatically through the years. WLE uses the full spectrum of visible white light to form a true to life representation of the mucosa. More recently the introduction of video chip technology has revolutionised the quality of image achievable, allowing for resolutions of over one million pixels. High Definition (HD) endoscopes use light from a xenon lamp, with the light reflected by the mucosa and detected by a HD charge coupled device located at the tip of the scope. Images can be enhanced further when combined with optical zoom lenses, which are capable of magnifying images by up to 150 times.1–4
Narrow band imaging
Narrow band imaging (NBI, Olympus, Japan) is probably the most widely available of the optical imaging techniques, incorporated into current generation Olympus video endoscopy systems. By using electronically activated filters it is possible to limit the wavelength spectrum of visible blue light to 415 nm and green light to 540 nm, while removing red light completely.5 These wavelengths coincide with the optimal frequency absorbed by haemoglobin, accentuating surface microvasculature and thereby providing enhanced delineation of the mucosal architecture. As angiogenesis is one of the first features of neoplasia, lesions appear darker than the background mucosa (figures 1 and 2). NBI is activated by simply pressing a button on the endoscope, allowing for rapid alternation between NBI and WLE images.5–7
Colonic polyp under white light endoscopy inspection.
Colonic polyp under narrow band imaging inspection, with clearer demarcation and characterisation of pit pattern compared with white light endoscopy.
Blue laser light imaging
Blue laser light imaging also known as Lasero (Fujinon, Japan) combines two lasers, one with a restricted wavelength spectrum of 415 nm, which highlights mucosal abnormalities using the same principles as NBI. The second laser provides fluorescent white light, enabling improved illumination of the mucosal surface, allowing for brighter images than achieved with traditional NBI. While this technology has been commercially available since 2012, data on its use is still emerging, with most studies having taken place in the Far East.
Fujinon intelligent chromoendoscopy
Fujinon intelligent chromoendoscopy (FICE, Fujinon, Japan) is a virtual chromoendoscopic technique, which, by using postprocessing algorithms, is able to digitally convert HD WLE images into colour images composed of various wavelength combinations (figures 3 and 4). The ten available presets can be customised from the many possible permutations, with an appropriate setting chosen on the basis of lesion characteristics.8
High grade dysplasia with white light endoscopy. Courtesy Dr Adolfo Parra Blanco.
High grade dysplasia with Fujinon intelligent chromoendoscopy (FICE) enhancement. Courtesy Dr Adolfo Parra Blanco.
I-Scan
I-Scan (Pentax Medical, Japan) similarly detects white light reflected by the gastrointestinal mucosa, converting this using postprocessing software to enhance lesion characteristics. I-Scan has three different modes; surface enhancement, contrast enhancement and tone enhancement modes. By augmenting light contrast, suppressing visible red light and enhancing blue light, it is possible in the various modes to provide topographical information and enhance mucosal vasculature9 (figures 5 and 6).
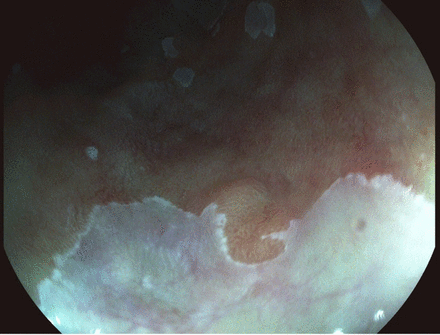
A subtle oesophageal lesion at 5 o'clock position, seen in white light endoscopy and proven to represent high grade dysplasia on biopsy. Courtesy Dr Adolfo Parra Blanco.
Enhanced visualisation of region of high grade dysplasia seen in figure 5, using I-Scan.
Autofluorescence imaging
Autofluorescence imaging (AFI) takes advantage of the differential presence of naturally occurring endogenous fluorophores within the gastrointestinal mucosa. When exposed to short wavelengths, fluorophores become excited and emit a longer wavelength of fluorescent light, with normal and abnormal tissues having different emission spectra.10 The placement of a rotating filter at the endoscope light source limits the excitation wavelength to blue light (390–470 nm) in order to induce autofluorescence and restricts green light (540–560 nm) for taking reflection images. A barrier filter placed at the AFI charge coupled device selectively detects fluorescent light of 500–630 nm thus eliminating the blue excitation. This results in an image composed of a mixture of green and mauve hues, which represent areas of normal and dysplastic mucosa, respectively (figure 7).10 ,11
Lesion within Barrett's oesophagus identified by autofluorescence imaging.
Confocal laser endomicroscopy
Confocal laser endomicroscopy (CLE) works by focusing blue laser light through a single lens onto a specific target. The reflected light is filtered through a pinhole, thereby reducing light scatter, creating highly detailed images from a thin focal plane.12 ,13 This is available as a miniature scanner integrated onto the endoscope tip or separately as a probe-based accessory fed through the working channel of a standard endoscope. Administration of an intravenous fluorescent contrast agent is necessary in order to achieve delineation of the subsurface architecture.12 ,13 CLE offers image detail comparable to histopathological sections (figures 8 and 9).
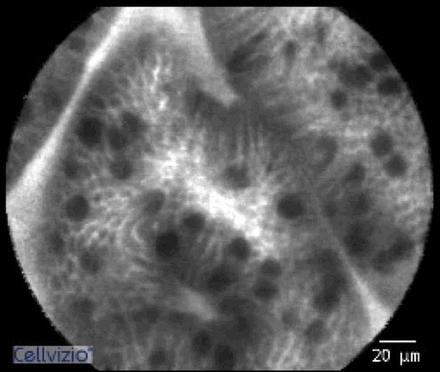
Probe based confocal laser endomicroscopy (pCLE) image of adenocarcinoma in Barrett’s oesophagus, demonstrating a pleomorphic irregular glandular structure.
pCLE image of intestinal metaplasia with dark mucin-laden goblet cells.
Evidence for advanced imaging within the gastrointestinal tract
Imaging in the oesophagus
Barrett's oesophagus has long been recognised as a precursor for oesophageal adenocarcinoma, with population studies suggesting an annual risk of progression of 0.33%.14 ,15 This has taken on greater significance in the advent of endoscopic treatments, such as radiofrequency ablation and endoscopic mucosal resection, which offer us the opportunity treat early lesions with improved patient outcomes.14 The standard of care is currently the Seattle protocol, with quadrantic biopsies taken at 2 cm intervals within the Barrett's segment. This technique is prone to sampling error, which could lead to inappropriate follow-up intervals.14
Several studies have pointed towards the benefits of using NBI to inspect Barrett's epithelium, a prospective trial in which patients were randomised to either WLE or NBI demonstrated comparable dysplasia detection rates, with nearly half the number of biopsies required.16 A meta-analysis demonstrated sensitivity and specificity of 96% and 94%, respectively, for the detection of high-grade dysplasia.17 Additional advantages of NBI include its availability outside of tertiary referral centres, as well as good interobserver agreement aided by the existence of NBI classification systems.18
AFI has shown initial promising results in increasing dysplasia detection but unfortunately is associated with a high false positive rate, which can be as high as 80%.11 ,14 To address this limitation, AFI tends not to be used in isolation, but rather is incorporated with NBI and HD WLE to create endoscopic trimodal imaging (ETMI). There have been positive outcomes when using ETMI to assess margins prior to endoscopic mucosal resection, with a small case series using this modality to achieve complete histological clearance in 87.5% of patients.19 A large randomised cross-over trial examining the impact of ETMI performed by general gastroenterologists with no specific expertise in Barrett's imaging in an intermediate risk group showed no significant improvement in dysplasia detection rates.20 Due to a combination of equipment cost, training requirements and lack of validation in low-risk populations, the use of AFI is currently limited to expert endoscopists in high-risk patients.11 ,14 ,20
Of the trials performed with CLE there is considerable heterogeneity in the technical aspects of its use, with variability in operator experience, use of a cap to reduce artefact, the type of CLE device, as well as the criteria used to make a diagnosis of neoplasia. Bearing in mind these limitations, a meta-analysis found the sensitivity of CLE in the detection of neoplasia to be 89%, with fewer biopsies required compared with WLE assessment.21 The operator requires training in CLE image acquisition and interpretation. CLE requires an additional capital investment in equipment and accessories that limits its widespread use.
At present there is insufficient evidence for the use of optical imaging techniques in preference of quadrantic biopsies for routine Barrett's surveillance.14 However, these have proven useful in high-risk patients, in whom quadrantic biopsies have detected the presence of dysplasia and in the context of treatment planning, where accurate delineation of lesion margins prior to resection is paramount.11 ,14 ,19
Imaging in the stomach
While gastric cancer is the third leading cause of cancer death worldwide, geographical variability, with a relatively low incidence in the West means algorithms for diagnosis and surveillance are not well established.22–24 Early gastric lesions tend to be subtle and can be easily missed with standard WLE, especially if coupled with a low index of suspicion. Vigilance for precursor conditions such as gastric atrophy, intestinal metaplasia (IM) and colonisation with Helicobacter pylori is required when patients attend endoscopy for alternate indications.25–27 At present, where atrophy is suspected it is recommended that biopsies are taken as per the Sydney protocol, with non-targeted biopsies within the antrum, incisura and fundus.27 ,28
HD WLE allows for the detection of IM and dysplasia with a sensitivity of 76% and 97%, respectively,29 while the use of magnification NBI has been shown to be accurate in differentiating between malignant and normal mucosa with a sensitivity of 97%.30 Further, changes on a cellular level, such as the presence of light blue crests that are indicative of IM can be visualised.31 A degree of experience in pathology recognition is required, with lower sensitivities for detection of IM by non-experts.31 While promising, it is noted that the majority of studies have taken place in Japan, where there is a greater incidence of gastric cancer and greater experience in lesion recognition. Further, NBI is often used in combination with high magnification endoscopes, which are not routinely available in daily clinical practice.31 It is uncertain whether such promising results are transferable to a low-risk population, with validation studies required.
The data on postprocessing methodologies is more limited, with the majority of the evidence based on postprocedural review of images. FICE has been shown to accurately delineate lesion margins as well as predict histological classification better than WLE alone. When used for assessment prior to endoscopic mucosal dissection FICE helps to achieve clear resection margins.32–35 Evidence for the use of I-Scan is still preliminary.
Studies on the use of CLE have demonstrated very promising results, in particular with a randomised controlled trial demonstrating far superior detection of IM with CLE of 65.7% compared with 15.7% with WLE. When combined with NBI there is 82% accuracy in histological classification of early gastric neoplasia; again, experience with this technology is required, with higher accuracies associated with expertise.36 ,37
Imaging in the colon
Colorectal cancer (CRC) remains the second leading cause of cancer mortality in the UK, with over 41 000 cases diagnosed annually and culminating in 16 187 deaths.38 ,39 The cumulative lifetime risk of CRC is in the order of 6%, however there is evidence that screening colonoscopy reduces incidence and mortality from CRC.40 Most sporadic CRCs develop from adenomas via the well described genetic adenoma-carcinoma sequence.41 Removal of adenomas at colonoscopy by polypectomy disrupts this progression. Countries that have embraced screening programmes have demonstrated significant reductions in the incidence of CRCs, with studies suggesting up to an 80% reduction in the subsequent development CRC in participants.41
Adenoma detection
Despite its benefits in the prevention of CRC, colonoscopy is limited by polyp miss rates of up to 26%.42 ,43 Consequently, the adenoma detection rate (ADR) is considered to be a highly discriminant indicator of colonoscopy quality. Although many factors may contribute to the polyp miss rate, it is thought that some of the lesions that are missed, particularly in the right colon, are flat and relatively subtle and therefore any improvement in the optics may make them easier to detect.43
Disappointingly, studies evaluating the use of HD WLE have shown only a modest improvement in ADR. A meta-analysis of five studies, comprising 4422 patients demonstrated a 3.5% (95% CI 0.9% to 6.1%) improvement in ADR as compared with standard WLE.44 There have been multiple large studies investigating the benefit of NBI, with some conflicting results. Two large meta-analyses have concluded that the use of NBI confers no additional benefit, with no difference in ADR with NBI compared with WLE.45 ,46 Some disadvantages of NBI in screening include the dark endoscopic image that results from poor illumination of the capacious colonic lumen, an effect that is compounded if combined with suboptimal bowel preparation. Even when NBI is limited to the right side of the colon, where it arguably should have greatest benefit, it failed to show an improved ADR in a multicentre Japanese study where 782 tandem WLE and NBI examinations of the right colon were performed.47 This is further supported by two trials where NBI was compared with WLE in a population at high risk for adenomas, with patients having either a positive faecal occult blood test or a history of previous polyps.48 ,49 There was no significant difference in the proportion of patients with adenomas, nor in the total number of adenomas identified. One explanation for the lack of additional benefit of NBI may be an improved skill at picking up polyps using WLE once trained in NBI use. The phenomenon of a ‘learning effect’ was seen in one study comparing WLE and NBI, where there was an initial greater ADR with NBI, which diminished as the study progressed as endoscopists became more adept at detecting these polyps with WLE.50 There is no convincing evidence for increased ADRs associated with the use of AFI, CLE, I-Scan or FICE.
Polyp characterisation
Diminutive polyps (<5 mm) rarely harbour advanced histology and it has therefore been suggested that such polyps need not necessarily be sent for histopathological assessment, but should rather be diagnosed at the time of colonoscopy based on polyp characteristics.51 ,52 This has the benefit of being able to determine an appropriate interval for further surveillance at the time of the procedure as well as potential cost savings in terms of histopathology and further follow-up.53 While this approach has yet to be validated by larger studies, it has been endorsed by the American and European Societies of Gastrointestinal Endoscopy for adequately experienced endoscopists.54 ,55
WLE alone is not good enough for accurate optical diagnosis and chromoendoscopy although comparable to histopathology in terms of accuracy, is cumbersome and has a long learning curve. Use of NBI for optical diagnosis has been widely examined and has proven accurate when performed by experts.51 ,56 A meta-analysis on the use of NBI for polyp characterisation, which included 28 studies with 6280 polyps, showed good concordance between endoscopic features and histopathological diagnosis.57 There was 91% accuracy for diagnosing neoplastic polyps, which when coupled with high confidence predictions resulted in an increased diagnostic accuracy. Somewhat disappointingly, several community based studies assessing optical diagnosis using NBI produced results significantly worse than those by experts, indicating that this technology is not quite ready for routine clinical practice.58 ,59
The use of FICE and I-Scan are comparable to the use of NBI, although fewer studies in these modalities mean with greater variability in diagnostic specificity and sensitivities. FICE has a sensitivity ranging from 73·9% to 100%, similar to I-Scan, which has a sensitivity ranging from 54·5% to 94·6%.60 ,61
Results from the trials studying the use of AFI are somewhat mixed. Where there is good differentiation between the green and purple areas on imaging, there is good correlation with histology. Unfortunately colour differentiation is often poor, creating ambiguity as to the significance of the imaged polyp and leading to a low specificity.62
CLE has proven to be highly sensitive (95%) and specific (94%) among 11 pooled trials and is the most promising of the imaging technologies. It is however worth noting the heterogeneity in the small number of trials conducted, with some basing results on postprocedure analysis of CLE images.62–65
Dysplasia detection in inflammatory bowel disease
The detection of preneoplastic lesions is especially important than in the context of underlying inflammatory bowel disease, where the incidence of CRC can be as high as 18% after 30 years of disease.66 Due to lesion subtlety the previous strategy for dysplasia detection was to survey the colon with non-targeted, segmental biopsies at 10 cm intervals. This biopsy protocol is not particularly efficacious with a yield of 0–0.2% per biopsy, with at least 33 biopsies required to achieve 90% certainty of detecting dysplasia if this is indeed present.67
Dye-based chromoendoscopy with methylene blue can increase dysplasia detection rates threefold to fourfold.68 ,69 Optical imaging aims to obviate the need for this cumbersome technique, with the greatest body of experience in NBI. To date NBI has been shown to be comparable to HD WLE combined with chromoendoscopy, rather than superior. Of the seven studies performed using NBI, most have had modest numbers of patients, consequently with small number of cases of dysplasia detected. Meaningful conclusions are difficult to draw. The largest of these studies was a multicentre trial, where 112 patients with ulcerative colitis (UC) were randomised to WLE or NBI that showed comparable dysplasia detection rates (9%), which was superior to segmental biopsies (0.04%).70 It is worth considering that studies of optical imaging usually use WLE as the standard for comparison, despite many guidelines suggesting WLE with dye-based chromoendoscopy as the gold standard surveillance technique.71
It is not yet clear whether AFI has a role in dysplasia detection in UC. A review of images taken with WLE and AFI showed a good correlation between AFI enhanced images and histologically confirmed mucosal inflammation.72 A study where tandem WLE and AFI endoscopies were performed in patients with UC demonstrated that AFI detected additional areas of dysplasia compared with WLE and demonstrated a polyp miss rate of 50% when using WLE compared with 0% with AFI.73 It is however noteworthy that in this study all patients had entirely quiescent disease, as differentiation between dysplasia and inflammation is often difficult with AFI. As in other areas of the gastrointestinal tract, AFI is associated with a high false positive rate.
To date CLE has demonstrated an impressive 97% accuracy in differentiating between dysplasia-associated lesions or masses and adenoma-like masses and is more sensitive in detecting dysplasia than WLE with dye-based chromoendoscopy. The time-consuming nature of this imaging technique means that it is unlikely to be pragmatic for standard surveillance.74–76
Emerging technologies
There are several imaging modalities that are currently being assessed, which are likely to play an important role in the future of in vivo diagnosis. Instead of enhancing mucosal abnormalities, these predominantly focus on early molecular change.
Optical coherence tomography
Optical coherence tomography (OCT) uses the same principles as B-mode ultrasonography to enable cellular imaging. Instead of using sound waves, near-infrared light is directed at the target tissue, with the differential light scatter detected and interpreted to create a cross-sectional image. Using light rather than sound creates images 10 times more detailed, with a scanning depth of up to 2 mm below the mucosal surface. OCT is available as a reusable probe, fed through the working channel of an endoscope.77 ,78
Variations of OCT, using different platforms, are likely to make this technology more accessible. Optical frequency domain imaging uses similar principles, with a rotating scanner to enable rapid acquisition of images within a large surface area.79 More recently, optical frequency domain imaging technology has been integrated within a tethered capsule, which can be swallowed by the patient, with images taken as this is drawn back through the oesophagus. An average of four passes, taking a total of 6 min, results in complete visualisation, without the need for traditional endoscopy.80 Meanwhile, volumetric laser endomicroscopy involves the inflation of a balloon within the oesophageal lumen. A scanning probe is pulled through the centre of this balloon, rotating 360° as it does so, thereby mapping the entire mucosa in contact with the balloon within 90 s.81
Spectroscopy
By interpreting the light scatter in response to projected light, it is possible to estimate the density of cellular components, by using spectroscopy. Early neoplastic change, such as increasing nucleus size and reduced cellular plasma can be detected. Spectroscopy encompasses a range of techniques broadly categorised into elastic and non-elastic, based on the retention of wavelength of the reflected light in the former, and the alteration of reflected wavelength in the latter.82 This probe -based imaging technology allows for submucosal visualisation without the need for contrast agents. These are, however, narrow field techniques, allowing for interrogation of a single point at a time and are therefore time-consuming.82
Molecular imaging
The field of molecular imaging offers the opportunity of identifying abnormal tissue by means of the differential expression molecules in health and disease. The use of glycans as a molecular target has been described in Barrett's oesophagus, with fluorescently labelled lectins used to highlight dysplastic tissue with high specificity.83
Conclusion
In selected situations advanced imaging techniques have been proven to be useful for optical diagnosis of gastrointestinal pathology. To date most of these methodologies have been used in the context of clinical trials or within tertiary hospitals with selected populations. Further studies are required to assess the utility of optical imaging in daily clinical practice. These techniques however, represent progress in terms of diagnostic capability as well as a paradigm shift in the role of endoscopy in patient management. Optical imaging is undoubtedly a useful addition in the armamentarium for the diagnosis of treatable early gastrointestinal pathology.
Acknowledgments
With thanks to Dr Adolfo Parra-Blanco for contribution of images.
References
Footnotes
Correction notice This article has been corrected since it published Online First. An Open Access licence has been added.
Collaborators Dr Adolfo Parra Blanco, Nottingham University hospital.
Contributors SB: primary author. AW: review and editorial of manuscript. KR: review and editorial of manuscript.
Competing interests AW: Olympus, Orion. KR has received consultancy, speaker honoraria, unrestricted research and educational grants from Olympus Keymed; research support from Mauna Kea, France; research grant and speaker honoraria from Pentax UK.
Provenance and peer review Not commissioned; externally peer reviewed.