Article Text
Abstract
Background In gastroenterological disorders, iron deficiency (ID) is often treated with intravenous iron. This real-world study assessed the effectiveness and safety of iron isomaltoside (IIM), a high-dose intravenous iron, for the treatment of ID in patients with gastroenterological disorders, as part of a service evaluation and improvement process.
Methods Medical records of 117 patients with gastroenterological disorders, who received IIM, were examined retrospectively. Study outcomes included dose of IIM (estimated iron need versus actual dose received), number of appointments required to deliver the dose and changes in haemoglobin (Hb) and ferritin at ~1 month and ~6 months post-treatment. Safety was assessed through adverse drug reactions (ADRs).
Results Overall, 76.1% of patients received their estimated iron need; 23.9% were underdosed. The mean (SD) iron dose was 1317 (409.7) mg; 62.4% of patients received their dose in one appointment. From baseline, mean (SD) Hb increased by 20.9 (15.4) g/L at 1 month post-treatment (p<0.0001) and by 22.0 (17.9) g/L at 6 months post-treatment (p<0.0001). Mean (SD) baseline ferritin was 26.6 (37.8) μg/L, which increased to 234.6 (142.9) μg/L at 1 month post-treatment (p<0.0001), and remained increased at 6 months post-treatment (122.8 (99.2) μg/L; p<0.0001). A substantial proportion of patients were non-anaemic at 1 month (57.5%) and 6 months (61.8%) post-treatment. At both post-treatment timepoints, the proportion of non-anaemic patients was higher in those receiving their total iron need versus those who were underdosed. No serious ADRs were reported.
Conclusion IIM was efficacious and well tolerated in patients with gastroenterological disorders. This real-world study highlights the importance of administering the full iron need to maximise treatment response.
- anemia
- IBD
- iron deficiency
Data availability statement
Data are available on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Significance of this study
What is already known on this topic
Iron deficiency (ID) is common in gastroenterological disorders and clinical guidance recommends intravenous iron for patients with iron deficiency anaemia (IDA) and clinically active inflammatory bowel disease (IBD).
Intravenous iron is also used to treat IDA in non-IBD disorders, although the management of these patients can be challenging due to a lack of available evidence from clinical trials conducted in specific patient populations.
Iron isomaltoside (IIM) is a high-dose intravenous iron with an established efficacy and safety profile demonstrated through randomised controlled trials.
What this study adds
Following an expected increase in ferritin at the first follow-up (~1 month after treatment), ferritin levels had subsequently decreased at the second follow-up timepoint (~6 months after treatment).
The mean estimated iron need for patients in this study was >1000 mg; however, some patients received less than their estimated iron need (ie, they were underdosed). Compared with underdosed patients, a higher proportion of patients who received their total estimated iron need were non-anaemic at first follow-up; this effect was maintained at 6 months.
IIM showed good effectiveness and safety for the treatment of IDA in a heterogeneous real-world patient population representing a wide range of gastroenterological disorders (IBD, gastric antral vascular ectasia), coeliac disease and Barrett’s oesophagus).
How might it impact on clinical practice in the foreseeable future
The ferritin data suggest that patients may be at risk of recurrent ID ~6 months after an intravenous iron infusion, advocating the need for reassessment of patients between 6 and 12 months after treatment.
The data from the present study support existing evidence that higher doses of intravenous iron lead to better haematological responses versus lower doses, which may translate into a reduced need for anaemia retreatment over time.
Introduction
Anaemia is common in patients with gastroenterological disorders,1 2 particularly in inflammatory bowel disease (IBD) where prevalence rates of up to 74% have been reported.3 Iron deficiency (ID) is one of the leading causes of anaemia in patients with gastroenterological disorders, resulting from reduced dietary intake of iron, impaired iron absorption or blood loss.1 4 5 The symptoms of ID (and anaemia) include fatigue, headaches, vertigo, impaired cognitive function and reduced physical performance, which have a negative impact on patient quality of life (QoL).1 6 Across various diseases, anaemia has also been associated with an increased risk of morbidity and mortality.7
Clinical guidance from the European Crohn’s and Colitis Organisation (ECCO) recommends intravenous iron therapy for patients with iron deficiency anaemia (IDA) and clinically active IBD.1 Oral iron is less effective in patients with gastroenterological disorders because intestinal inflammation results in decreased iron absorption,8 and the gastrointestinal side effects of oral iron are often intolerable.7 9 Intravenous iron is also used to treat IDA in gastroenterological disorders other than IBD (eg, gastric antral vascular ectasia (GAVE), coeliac disease and cancer), where patients are intolerant of, or unresponsive to, oral iron.5 10 Given the chronic nature of some gastroenterological disorders—IBD in particular—patients can experience considerable iron losses and are likely to require repeated courses of intravenous iron to treat recurrent anaemia.11
Iron isomaltoside 1000/ferric derisomaltose (IIM) (produced by Pharmacosmos A/S, Denmark; marketed as Monofer/Monoferric) is a high-dose intravenous iron preparation that has demonstrated effectiveness and safety in clinical trials and observational studies for the treatment of ID/IDA in patients with gastroenterological disorders.12–18 IIM is approved in Europe for fast delivery of doses ≤20 mg/kg body weight in a single visit.19
As part of a service evaluation and improvement process, the present study assessed the effectiveness and safety of IIM for the treatment of ID/IDA in patients with gastroenterological disorders.
Methods
Patient population
The medical records of patients with gastroenterological disorders who had received intravenous IIM for the treatment of ID/IDA at Antrim Area Hospital, Northern Ireland, between January 2016 and May 2019 were examined retrospectively.
Patients with ID or IDA were eligible to receive IIM according to local guidelines if oral iron was contraindicated (eg, in cases of gastric irritability) or if they were intolerant of oral iron, had active IBD or had severe anaemia. In patients with active IBD, ID was defined as having a ferritin level >100 µg/L and a transferrin saturation <20%; absolute ID was defined as a ferritin level of <30 µg/L. Patients were considered to be anaemic if the haemoglobin (Hb) level was <130 g/L for men and <120 g/L for women; an Hb level <90 g/L indicated severe anaemia.
IIM was administered using a local treatment protocol that was developed based on the ECCO guidelines. A simplified dosing regimen was used to estimate the patient’s iron need (table 1). However, iron dosing was not always consistent, particularly for patients with a body weight <50 kg. If the total iron need was >20 mg/kg, the full iron dose was administered at two separate appointments, ~1 week apart.
Haematinics (Hb and ferritin) were documented following IIM treatment at two follow-up timepoints: ~1 month post-treatment and ~6 months post-treatment.
Simplified dosing regimen for the estimation of iron need19
Data collection and outcomes
Data on patient demographics, IIM dose, haematinics and adverse drug reactions (ADRs) were collected from patient medical records. The study evaluated the dose of IIM (estimated iron need vs actual dose administered) and the number of appointments required to deliver the total prescribed dose. Additional outcomes assessed at the first and second follow-up timepoints were the changes from baseline in haematinics, the proportion of non-anaemic patients (ie, those with an Hb level ≥130 g/L (males) and ≥120 g/L (females)) and response rate (defined as the proportion of patients who were non-anaemic or experienced an Hb increase of ≥20 g/L from baseline). Anaemia status in patients receiving their estimated total iron need versus those who received less than their total iron need was evaluated. Estimated total iron need was calculated using the simplified dosing method (table 1), based on individual patient baseline Hb and weight. The proportion of patients retreated with intravenous iron was also recorded.
Statistical analyses
Changes from baseline in haematinics at both follow-up timepoints were analysed using a two-sided, one-sample t-test. At both follow-up timepoints, the difference in the proportion of non-anaemic patients was compared between the group who received their total iron need and those who received less than their total iron need using a Fisher’s exact test. Descriptive statistics were used to analyse safety results.
Ethics
The study was approved by the local audit department, as part of a service evaluation for improvement in the implementation and assessment of intravenous iron therapy. The study adhered to the principles of the Declaration of Helsinki, International Council of Harmonisation and Good Clinical Practice guidelines.
Results
Patient population
Baseline demographics and clinical characteristics were collected for 117 patients (table 2). The study population was mostly females and represented patients with IBD and non-IBD disorders (including GAVE, coeliac disease and Barrett’s oesophagus (online supplementary table S1)). Baseline haematinic data showed that patients had ID and that the vast majority were also anaemic. Oral iron (ferrous fumarate) had previously been prescribed to almost one-third of patients, many of whom reported intolerance. Baseline data for the non-IBD group show no difference versus the IBD group (online supplementary table S2).
Supplemental material
Baseline demographics and clinical characteristics
Iron dosing
Across all patients with Hb and weight data (n=113), the mean (SD) actual dose of iron received was 1317 (409.7) mg. The mean (SD) estimated iron need calculated during the data analysis was 1385 (438.2) mg; 86 (76.1%) patients received their estimated iron need, while 27 (23.9%) patients received less than their estimated iron need (table 3).
Estimated iron need versus actual iron received
If the patient’s iron need exceeds 20 mg/kg, the dose of IIM should be administered in two separate appointments.19 The total prescribed IIM dose was administered in one appointment for 73 (62.4%) patients, while 44 (37.6%) patients required a second appointment. Of the 59 patients prescribed a cumulative dose of >1000 mg of iron, 18 were treated in one appointment.
Effectiveness
Following IIM treatment, the mean (SD) time to the first follow-up was 44 (46.4) days, and to the second follow-up was 179 (71.8) days.
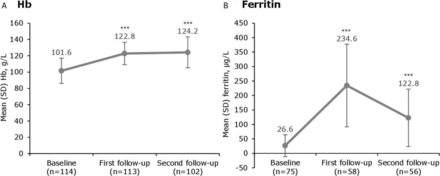
Compared with baseline, the mean Hb was increased by 20.9 g/L at the first follow-up (p<0.0001) and by 22.0 g/L at the second follow-up (p<0.0001) (figure 1A). The corresponding median (range) values were 124 (84–162) g/L and 126 (73–161) g/L, respectively.
Haematinic parameters before and after treatment with iron isomaltoside 1000. ***p<0.0001 versus baseline. Median (range) time to first follow-up was 30 (3–388) days and to second follow-up was 181 (27–513) days. Hb, haemoglobin.
As expected, the mean ferritin level increased by the first follow-up (p<0.0001 vs baseline). At the second follow-up, the mean ferritin level decreased from the previous assessment, but remained significantly higher compared with baseline (p<0.0001) (figure 1B). The median (range) ferritin level was 199.5 (13–585) µg/L at the first follow-up and 90.5 (12–452) µg/L at the second follow-up.
A substantial proportion of patients (57.5%) were non-anaemic at the first follow-up, increasing to 61.8% at the second follow-up (figure 2A). At both timepoints, the proportion of non-anaemic patients was higher in the group who received their total iron need compared with the group who received less than their total iron need (figure 2B). The response rate was 73.5% at both follow-up timepoints.
Proportion of patients who were non-anaemic after treatment with iron isomaltoside 1000. The difference in the proportion of non-anaemic patients was compared between the group who received their total iron need and those who received less than their total iron need using Fisher’s exact test—the difference was not statistically significant at either timepoint. Non-anaemic status was defined as a haemoglobin level of ≥130 g/L for males and ≥120 g/L for females. Median (range) time to first follow-up was 30 (3–388) days and to second follow-up was 181 (27–513) days.
A total of 27 (23.1%) of patients who received IIM were retreated with intravenous iron within 24 months (table 4). The exact date of retreatment was documented for nine patients; of these, only two patients had been retreated within 6 months of receiving IIM.
Retreatment with intravenous iron
Safety
Three (1.9%) ADRs were reported in 160 IIM infusions. These were recorded as infusion reactions, including one case each of: blisters on the arms and trunk, nausea and retching, and lower back pain. The patient who experienced blisters received their full IIM dose and was treated with intravenous hydrocortisone/antihistamine. The patient with lower back pain was successfully rechallenged with IIM and received their full dose. No ADRs of serious/severe hypersensitivity reactions occurred and no intervention with epinephrine was required. All three patients recovered without sequelae.
Discussion
This service evaluation examined the real-world use of IIM for the treatment of ID/IDA across a range of gastroenterological disorders. IIM demonstrated a good effectiveness and safety profile, successfully treating ID/IDA in this patient population, in line with previous observational studies and clinical trials in patients with gastroenterological disorders.12–18
The study population included patients with a range of gastroenterological disorders and, therefore, it is representative of a typical cohort attending a gastroenterology intravenous iron clinic, where ID/IDA is common. However, ID/IDA management in these patients is challenging due to a lack of robust evidence from clinical trials conducted in patients with non-IBD disorders. In the present study, substantial and clinically relevant improvements in Hb (an increase of ≥20 g/L) and ferritin were observed after IIM administration, confirming that IIM is effective in this heterogeneous population. By treating patients with IIM, intolerance of oral iron in certain patients with non-IBD disorders was no longer an issue and, although not formally assessed, these patients reported improvements in their QoL and treatment satisfaction. Further studies are necessary to better understand anaemia in patients with non-IBD disorders and to identify the optimal treatment regimen.
At the first follow-up timepoint, ~1 month after IIM treatment, a substantial increase in the mean Hb level (>20 g/L) was observed. This is in line with previous studies of IIM (and other intravenous iron preparations) in similar patients populations in real-world settings,16 20 and also with the definition of an acceptable treatment response outlined in the ECCO guidelines.1 The increase in Hb was maintained at the second follow-up, ~6 months after treatment, further confirming the effectiveness of IIM. These data support the findings of Frigstad et al (2017) who reported that IIM administered in high doses significantly improved Hb levels in patients with gastroenterological disorders and ID.16 In the Frigstad study, the haematological benefits of IIM translated into a reduced need for anaemia retreatment over a 12-month period.16
In line with previous studies investigating the treatment of ID in patients with IBD, the present study observed increases in ferritin at the first follow-up, which decreased at the second follow-up.12 15 21 This decrease in ferritin was, presumably, the result of iron being used during haematopoiesis as reflected in the sustained increase in Hb; alternatively, the decrease may have been due to further blood loss.1 22 Whichever the cause, the ferritin data suggest that patients may be at risk of recurrent ID and support the need for patient reassessment at 6–12 months post-treatment. The potential for iron overload following treatment with intravenous iron is a concern for patients with IBD and for those with other primary diseases, such as chronic kidney disease (CKD).23 However, in the present study, the maximum ferritin level recorded was 585 µg/L. Even accounting for elevations in ferritin levels caused by inflammation, the values recorded are lower than the threshold of 800 µg/L that is considered to be toxic, and which should be avoided.1 24 25
The mean estimated iron need for patients in this study was 1385 mg, in accordance with the evidence from large randomised controlled trials (RCTs) indicating that the iron requirement in patients with gastroenterological disorders often exceeds 1000 mg.12 21 26 Nevertheless, some underdosing was observed in this study, which appeared to occur in the first patients treated with IIM. This may reflect habitual prescribing by clinicians, as the single maximum dose of the intravenous iron therapy previously used was 1000 mg. Compared with patients who experienced underdosing, a higher proportion of patients who received their total estimated iron need were non-anaemic at ~1 month and at ~6 months. Although the difference between these two groups did not reach significance in our study, this observation is consistent with the findings of a similar real-world study in patients with ID and heart failure—patients who were dosed optimally, according to validated methods, showed better functional and biochemical improvements.27 Taken together, the data from the present study and other real-world evaluations support existing evidence that higher intravenous iron doses lead to better haematological responses versus lower doses15 16 21 and can reduce the need for anaemia retreatment over time.16
Most patients were non-anaemic by the second follow-up. However, some patients remained anaemic at this timepoint, which could be due to various reasons. For example, some patients may have received less iron than their total estimated need, and others may have required even higher amounts of iron. In addition, some patients may have experienced post-treatment bleeding, which is not uncommon in this patient cohort. Furthermore, there was wide variation in the timings of the follow-up assessments—the time interval between intravenous iron treatment and the second follow-up was 27–513 days and some patients may have experienced a recurrence of anaemia during this time. In the present study, 23% of patients were retreated with intravenous iron within 24 months of their initial IIM infusion. Although the specific need for retreatment was not documented, it is possible that some of the patients who were still anaemic at ~6 months contributed to this proportion.
In the present study, >60% of patients were treated in one appointment. Minimising the number of appointments required is important as it reduces the burden on patients and alleviates pressures on healthcare. In fact, it has been previously reported that IIM can provide cost savings to the healthcare system through lower resource utilisation, compared with other intravenous iron preparations.28 29
No events of serious/severe hypersensitivity were reported. The safety data are consistent with RCTs and other real-world studies showing a low ADR rate with IIM.12 13 15 30 31 Sivakumar et al (2019) reported an ADR rate of 0.54% in a large real-world study of >1100 IIM administrations in non-dialysis-dependent patients with CKD in the UK.31 Published evidence, together with the data from the present study, demonstrate that modern intravenous irons are well tolerated and support the finding that intravenous irons show lower hypersensitivity rates than other widely used intravenous medications.32 33
Considering the study limitations, data were collected retrospectively from the records of a relatively small number of patients. Follow-up data for iron parameters and other potentially relevant laboratory parameters were not available for all patients. Furthermore, the patient population was heterogeneous and data from the different subpopulations were not analysed separately, meaning that the conclusions may not be applicable to each specific gastroenterological disorder or to anaemic and non-anaemic patients, independently. However, it is important to note that this was an observational study, conducted as part of an improvement process and designed to evaluate a real-world intravenous iron treatment service for gastroenterological disorders; it was not designed to address any specific hypotheses as would be expected from a clinical trial.
Conclusions
This anaemia service evaluation confirms the real-world effectiveness and safety of IIM for the treatment of ID/IDA in patients with gastroenterological disorders. This study supports existing findings that patients with gastroenterological disorders have a high iron need. Ideally, patients should be systematically monitored during the 6- to 12-month period following intravenous iron treatment to capture a decrease in ferritin levels, and prompt a consideration of the need for retreatment to prevent the recurrence of ID/IDA.
Data availability statement
Data are available on reasonable request.
Ethics statements
Acknowledgments
We thank the patients and infusion staff at the Gastroenterology Department of Antrim Area Hospital, and especially Louise Scullion for assisting with data collection. We are also grateful to the Consultant Gastroenterologists at Antrim Area Hospital for their kind collaboration, and to Pharmacosmos UK Ltd for partly supporting this study. We also acknowledge Cambridge Medical Communication Ltd (Cambridge, UK) for providing the writing support and Pharmacosmos UK Ltd for funding.
References
Footnotes
Contributors Both authors were involved with the concept and design, the analysis and interpretation, the drafting of the paper and revising it critically for intellectual content. The authors also approved the final manuscript and agree to be accountable for all aspects of the work.
Funding Pharmacosmos UK Ltd provided a grant that partly funded this project.
Competing interests JK has received speaker honoraria and consultancy fees from Pharmacosmos UK Ltd.
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- UpFront