Article Text
Abstract
Gastroenterologists are intermittently involved in diagnosing and managing patients who have neuroendocrine tumours (NETs). However, few UK gastroenterologists have received extensive training about this topic. This article aims to provide a brief introduction to NETs; it is aimed at a general gastroenterologist audience.
NETs present in diverse ways and many symptomatic patients unfortunately experience significant delays in diagnosis. Comprehensive evaluation of a patient with a possible NET involves assessing their symptoms, the tumour’s primary organ of origin, its differentiation status, grade and stage, whether the NET is secreting hormones and whether there is any underlying hereditary predisposition. Such assessment often needs specialist investigations such as nuclear medicine scans. All these factors influence patient management and prognosis, so a patient’s case and investigations should always be discussed by a fully constituted NET multidisciplinary team. Most localised tumours are considered for resection, but there are multiple treatment options for metastatic disease and many patients receive several different therapies during the course of their illness. The most common first line treatment in patients who have metastatic low grade NETs is monthly long acting somatostatin analogue injections. Prognosis is highly variable, but some patients who have inoperable metastases survive for many years on treatment with good quality of life. Gastroenterologists may also be involved in managing the non-tumour associated chronic gastrointestinal problems that some patients experience. Their involvement has been shown to improve patient-reported outcomes and quality of life.
- gastrointestinal neoplasia
- gut hormones
- gastrointestinal cancer
- small bowel disease
Statistics from Altmetric.com
Introduction—NETs are not as rare as you may think
Neuroendocrine tumours (NETs) comprise a diverse set of relatively rare neoplasms. They arise at several locations within the body, but many of the most common primary sites involve the gastrointestinal (GI) tract and pancreas (hence the term gastroenteropancreatic (GEP)-NETs). GEP-NETs are therefore encountered periodically by most gastroenterologists during their routine clinical practice, but most of these clinicians would not consider themselves to be experts in this field. The aim of this article is to demystify the topic, so that practising general gastroenterologists can arrange appropriate initial investigations and can provide accurate information to their patients while they are being referred to NET specialists based at their regional multidisciplinary team (MDT). Its content has been influenced by the topics that were discussed by participants at a Frontline Gastroenterology Twitter debate on this topic in December 2019. The article is not aimed at clinicians who are already providing specialist care to NET patients and nuances of tertiary level care are beyond the scope of this review. We assume that definitive investigations and management will be undertaken by a specialist NET MDT.
Although once considered rare, a recent epidemiological study reported a 6.4-fold increase in NET incidence between 1973 and 2012. This analysis of the US-based surveillance, epidemiology and end results program (SEER) database reported an annual age-adjusted incidence of 6.98 per 100 000 persons.1 At least some of this increase in incidence is likely to have resulted from heightened awareness of the tumour type and enhanced detection by modern imaging and endoscopic techniques, but an actual real increase may also have contributed. As many patients who have NETs experience prolonged survival, even when they have metastatic disease (median overall survival 9.3 years), Dasari et al also reported a 20-year limited duration prevalence of 0.048%.1 This prevalence (approximately 35 per 100 000) is higher than that of any other tumour arising within the GI tract with the exception of colorectal adenocarcinoma. A similar increase in incidence has also recently been reported in England, where the current age-standardised incidence is 8.6 per 100 000 (4.6 per 100 000 for GEP-NETs).2 Recent Cancer Research UK Statistics suggest that this overall UK age-standardised incidence for NETs is only slightly lower than that of other cancers that many gastroenterologists assume to be more common such as gastric adenocarcinoma or hepatocellular carcinoma. These statistics imply that most general gastroenterologists will intermittently diagnose new cases of NET and, in addition, they are likely to regularly encounter patients who have established NET diagnoses.
Terminology and classification systems for NETs
One potentially confusing aspect of the NET field is the terminology that is employed, as this has changed substantially over recent years. The term neuroendocrine neoplasm (NEN) is now used to refer to all tumours of this type. Many of these tumours are well-differentiated and slow growing with a relatively good prognosis (termed NETs, table 1), whereas a minority are poorly differentiated and faster growing with a relatively poor prognosis (termed neuroendocrine carcinomas (NECs), table 1). Historically, NETs used to be described as ‘carcinoid tumours’, but in most countries, including the UK, this term is no longer used except for the NETs that arise in the lungs (bronchial carcinoids) and as part of the term ‘carcinoid syndrome’ (see below). Although technically inaccurate, we have sometimes used the abbreviation NET in this paper to refer to all NENs, as it is currently the more generally employed term.
Classification of neuroendocrine neoplasms according to pathological differentiation and grade according to Ki-67 proliferation index
NETs can arise throughout the GI tract and pancreas, but the most common sites are the small bowel, rectum, pancreas, stomach and appendix in decreasing order of frequency.1 Tumour site is a major determinant of prognosis. For example, most appendiceal NETs have a very good prognosis. Tumour size is also an important prognostic factor for localised NETs at most sites.
The most important feature in establishing a GEP-NET diagnosis is histology. This not only establishes the tumour type, but also influences treatment decisions and prognosis. Tissue for histopathological evaluation can be obtained via endoscopic or radiologically guided biopsy or by surgical resection depending on the tumour site. A crucial feature is the establishment of a tumour’s grade (which is an indication of the proportion of NET cells that are proliferating at a particular time) by Ki-67 immunohistochemistry or mitotic count. NETs are currently classified as grades 1, 2 and 3 using Ki-67 cut-offs of <3%, 3%–20% and >20%, respectively. Grade 3 neoplasms are additionally subclassified into those which show well-differentiated histology (NETs) and those which demonstrate poorly differentiated histopathological features (NECs) (table 1).
In addition to establishing a tumour’s grade, tumour stage should be determined using conventional cross-sectional radiological imaging (CT and/or MR scan) and in many patients functional imaging techniques (see below). There are specific detailed tumour, lymph node, metastasis (TNM) staging systems for NENs that arise at different anatomical sites, but as for most other GI tumours, stage 1 and 2 NENs are confined to the organ of origin, stage 3 NENs have lymph node metastases and stage 4 NENs have distant metastases (most commonly in the liver and bones). It is important to note that in contrast to most other GI cancers, some patients who have widespread stage 4 NETs have a relatively good prognosis, especially when the tumour is grade 1.
The complete evaluation of a patient who has a NET also involves determining whether the tumour is secreting hormones or other bioactive compounds (ie, whether it is functional (secretory) or non-functional (non-secretory)). Functional tumours result in hormonally induced syndromes (eg, carcinoid syndrome due to serotonin secretion from a metastatic small bowel NET or Zollinger Ellison syndrome due to gastrin hypersecretion from a pancreatic gastrinoma). The biochemical tests that are used to establish functionality are discussed below.
Finally, consideration should be given as to whether there is any potential underlying reason why a patient has developed a GEP-NET. For example, some pancreatic NETs develop because a patient has a genetically predisposing condition such as multiple endocrine neoplasia type I, von Hippel Lindau syndrome or neurofibromatosis type 1. Detecting the presence of one of these hereditary conditions has implications for therapy (eg, it may affect the time at which pancreatic surgery is performed) as well as for screening other family members.
Complete characterisation of a NEN therefore involves extensive investigations in order to establish its organ of origin, grade, differentiation status, stage, and functional status as well as the presence of any potential hereditary predisposing condition (eg, the final diagnosis may be one of a sporadic non-functional, well differentiated, grade 1, stage 4 terminal ileal NET).
Clinical presentation
A small proportion of NET patients present with specific symptoms such as those caused by carcinoid syndrome (the classical triad of diarrhoea, flushing and wheezing). However, a much larger number of patients, particularly those with non-functional tumours, present with less specific symptoms such as change in bowel habit and/or abdominal pain. Some patients are even entirely asymptomatic and their NET is detected incidentally while they are undergoing a scan or endoscopy to investigate an unrelated indication. The diagnosis of patients with NETs can therefore be very challenging.
Despite a general increased awareness of GEP-NETs by clinicians, many patients still unfortunately experience considerable delays in diagnosis. This is often due to the non-specific nature of the symptoms that are often associated with GEP-NETs and the frequent overlap between these symptoms and those of other much commoner and less life threatening conditions such as irritable bowel syndrome (IBS). This may also be influenced by a perceived pressure in some cases to discharge patients with such symptoms who have a normal gastroscopy and/or colonoscopy. A recent international survey reported a mean patient-reported time from first symptom onset to NET diagnosis of 52 months3; while a recent survey that was initiated within the UK reported a median duration from the time of first symptoms to diagnosis of 36 months for small bowel NETs and 24 months for pancreatic NETs.4 Clinical features that may suggest a need for further investigations such as contrast-enhanced abdominal CT scan may include new onset of symptoms in older patients in whom a new diagnosis of IBS is less likely, persistent symptoms (eg, diarrhoea or abdominal pain), the presence of facial flushing or weight loss or any features suggestive of bowel obstruction.
When NETs are strongly suspected on the basis of symptoms (eg, features of carcinoid syndrome) or radiological investigations or when they have been diagnosed incidentally following histology, patients should be referred to a specialist NET MDT for comprehensive investigations and definitive management.
Rational investigations
Endoscopy
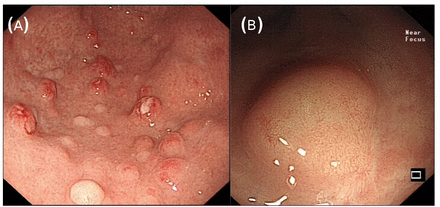
GEP-NETs are frequently detected incidentally during upper or lower GI endoscopy performed for other indications (figure 1). The number, size and site of polyps should be recorded with capture of photographic imaging according to the British Society of Gastroenterology quality standards.5 With gastric NETs, biopsies from surrounding normal gastric antral and corpus mucosa are essential to determine the type of gastric NET, the majority being type I gastric NETs associated with autoimmune atrophic gastritis and frequently pernicious anaemia with type III gastric NETs having the worst prognosis (table 2).6 The majority of duodenal NETs diagnosed at endoscopy are non-functional. In a suspected duodenal gastrinoma, biopsies of gastric mucosa together with pH measurement of gastric aspirate can be useful. Ideally endoscopy should be performed by a gastroenterologist with experience of NETs. Endoscopic polypectomy of gastric and duodenal NETs may need specialist advanced techniques such as endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) recognising the risk of perforation/haemorrhage (especially in the duodenum) and the appropriateness of ‘watch and wait’ in some cases.6 Endoscopic resection should be undertaken as a planned procedure rather than at index endoscopy. This should not be undertaken in the small bowel where surgical resection is recommended instead. Often, small rectal NETs (with a yellow tinge and normal pit pattern) (figure 1) can be misidentified as hyperplastic polyps where simple snare polypectomy may not be appropriate. Endoscopic ultrasound can therefore sometimes be helpful, especially with rectal NETs.7 Rectal NET management is generally dependent on size as to whether endoscopic or surgical resection is performed according to European Neuroendocrine Tumor Society (ENETS) guidelines (figure 2).7
Gastric NET classification and features
Endoscopic images of (A) multiple type I gastric NETs with a background of atrophic gastritis and (B) a solitary rectal NET. NET, neuroendocrine tumour.
Algorithm for management of rectal NETs (adapted from ENETS Consensus Guidelines7). EUS, endoscopic ultrasound; G, grade; NET, neuroendocrine; PET, positron emission tomography; SRS, somatostatin receptor scintigraphy; TME, total mesorectal excision.
Imaging
After establishing a diagnosis of NET, most patients undergo cross sectional imaging of the thorax, abdomen and pelvis using CT and/or MRI. In addition, an 111In-octreotide scan or the more sensitive (and currently less generally available in the UK) 68Ga-DOTATATE PET-CT scan may be helpful (figure 3). These functional imaging techniques are sometimes more sensitive for detecting the presence of metastases than conventional cross sectional imaging and additionally establish the somatostatin receptor status of the tumour. The latter is important for determining the suitability of some therapies. FDG-PET-CT scans can also sometimes be useful for staging, especially those patients with grade 3 NENs. Additional investigations to establish whether a NET is causing an extra-intestinal manifestation, such as echocardiography to determine whether a patient with carcinoid syndrome has carcinoid heart disease may also be indicated in certain patients.
68Ga-DOTATATE PET/CT scan demonstrating a large pancreatic NET with liver and nodal metastases as well as a bone metastasis in the right iliac crest. Note there is also physiological tracer uptake in the spleen, kidneys, bladder and pituitary gland. NET, neuroendocrine tumour; PET, positron emission tomography
Biochemistry
Biochemical tests are useful for establishing the functional status of a NET. However, in view of their specificity and sensitivity, they are of very limited use as a screening test for the presence of NETs, and their use in this setting is therefore not advocated. The serum/plasma concentration of the relatively non-specific biomarker chromogranin A is measured in most patients, but levels may be normal especially in small localised NETs and gastroenterologists should also be aware that conditions such as renal failure, inflammatory bowel disease (IBD) and proton pump inhibitor use can sometimes cause false positive elevations. The fasting gut hormone test establishes the plasma concentrations of gastrin, somatostatin, glucagon and vasoactive intestinal peptide and is helpful for characterising (mostly pancreatic) NETs. False positive elevations are, however, common, especially elevated gastrin concentrations as a result of acid suppressant medication use. Finally, the 24 hours urinary (or in some hospitals plasma) concentration of 5-hydroxyindoleacetic acid (5-HIAA) can be helpful in evaluating a patient who potentially has a metastatic small bowel NET and/or suspected carcinoid syndrome. However, gastroenterologists should again be aware of the multiple causes (including dietary) of false positive elevations of this test. Finally, the concentrations of NT-proBNP should be monitored in patients who have carcinoid syndrome as this is a useful biomarker for the presence of carcinoid heart disease.
Treatment
Principles of treatment
The management of patients with NETs can be extremely complicated and ideally should take place after discussion in a regional NET MDT according to UK and Ireland Neuroendocrine Tumour Society (UKINETS)/ENETS guidelines. Factors determining management include site of primary tumour, stage, grade, functionality of the tumour, site of metastases, availability of specialised investigations and treatments/surgery, patient fitness and choice, age and comorbidities. Management algorithms are too complex for this review due to the heterogeneity of NETs, but ENETS and UKINETS have produced guidelines with comprehensive flowcharts.8 9
In principle, if a NET is localised and resectable, it should usually be resected (apart from small non-functional duodenal NETs in the elderly, small non-functional pancreatic NETs, type I gastric NETs <15 mm and some others, where there may be an argument for adopting a ‘watch and wait’ policy if tumours are also low grade). Even if a patient has a good prognosis and is relatively asymptomatic, but has a slow growing, low grade, localised small bowel NET, oncological resection can reduce the risk of desmoplasia in the future, and thus avoid potential inoperable small bowel obstruction and chronic GI symptoms. Resection of small bowel tumours in particular should also be considered even in the presence of metastases. Obviously, life expectancy must be taken into consideration. If a NET is functional, particularly with the carcinoid syndrome, somatostatin analogue injections (lanreotide autogel or octreotide long-acting release which bind to somatostatin receptors) every 4 weeks reduce the symptoms that are caused by secreted peptides.8 9 Hepatic artery embolisation and telotristat, a new tryptophan hydroxylase inhibitor10 can also sometimes be useful for resistant symptomatic cases.
Management of metastatic disease
Somatostatin analogues are also the mainstay of treatment in the more common non-functional, unresectable, locally advanced or metastatic NETs of low/intermediate grade. Large randomised controlled trials, CLARINET11 and PROMID,12 have demonstrated delayed tumour progression with these agents. Ideally, a 111In-octreotide scan or 68Ga-DOTATATE PET-CT scan is required to ascertain somatostatin receptor positivity prior to treatment. There is a role for hepatic resection (and/or radiofrequency ablation/embolisation) for synchronous or metachronous liver metastases in low or intermediate grade NETs in certain situations, but this should be discussed in a centralised NET MDT.8 9 Otherwise, the mainstay of treatment for locally advanced or metastatic disease is somatostatin analogues and a choice of chemotherapy or the tyrosine kinase inhibitors sunitinib or everolimus for the less common higher grade tumours, especially pancreatic NETs which are generally more aggressive. While on treatment patients usually undergo imaging at intervals between 3 and 12 months to monitor for progression according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria.
For midgut and pancreatic NET patients who have positive 111In-octreotide or 68Ga-DOTATAE PET-CT scans, if radiological progression is detected during first line treatment, peptide receptor radionuclide therapy is an effective and well-tolerated second line treatment option.13 This is given as several infusions over a year and consists of a somatostatin analogue labelled with a radioactive component (177Lutetium-DOTATATE) that binds to somatostatin receptors, delivering the radioactive element internally to NET cells.
Metastatic poorly differentiated tumours (NECs) are generally treated with platinum based chemotherapy (with palliative intent), but overall, unlike other cancers, chemotherapy is not very effective in the commoner lower grade and well-differentiated NETs.14
Determinants of prognosis
There are a number of factors which have been demonstrated to be prognostic in NET patients in addition to age and comorbidities. It is impossible to give a general overall prognosis for NETs due to their heterogeneity. Some patients with aggressive NECs only survive a few months from the time of diagnosis, while some patients who have stage IV metastatic small intestinal NETs can survive two decades with good quality of life on treatment.1 The site of primary tumour is important with type I and II gastric, appendiceal and rectal NETs having excellent prognosis at similar stages compared with small intestinal and pancreatic NETs. NETs in the rectum appear to have the best prognosis, while NETs in pancreas have the highest risk of mortality. The US SEER data demonstrate HRs using the rectum with a median overall survival of 66 months as a reference of: pancreas median survival 27 months, HR, 2.034; small intestine, HR, 1.660; stomach, HR, 1.865).15
Grade as determined by Ki-67% has repeatedly been shown to be prognostic with lower grade NETs having a better prognosis than higher grade NETs.16 Stage is also prognostic with metastatic disease having worse prognosis than localised disease.16 The US SEER data suggest a median overall survival of 54 months for localised disease compared with 10 months for distant disease for all NENs.1 However, compared with other GI cancers, several patients can have a relatively good prognosis on treatment even with widespread stage IV disease.
The role of a gastroenterologist in managing NETs
Gastroenterologists have a large part to play in the diagnosis and management of patients with NETs alongside other key specialities such as oncology, endocrinology, surgery, pathology, radiology, nuclear medicine and specialist nurses as part of an MDT. The delayed diagnosis often found (24–36 months4) in particular with GEP-NETs (which comprise the majority of NETs) is often due to alternative diagnoses initially being made including IBD and IBS and is a worldwide problem without much improvement. Referral pathways suggest that gastroenterologists and gastrointestinal surgeons are the predominant specialities making the diagnosis of NETs with increased awareness and education of these specialities being shown to lead to a reduction in diagnosis time in Wales.17 Many patients who have GEP-NETs essentially have a chronic GI and hepatic disease with symptoms, ‘flares’ and changes in imaging indicating a need for changes in management or surgery, analogous to models of care in IBD. Some patients have liver metastases and treatments that are used overlap with those used in hepatocellular cancer. Finally, during or after treatment, many patients experience chronic GI symptoms from a number of causes which can impact quality of life. These may involve bile acid malabsorption, pancreatic insufficiency or small intestinal bacterial overgrowth and can be improved by the involvement of a gastroenterologist.18 The addition of gastroenterologists as core members of the NET MDT is advised by ENETS and can improve patient outcomes,18 but unlike some European countries, gastroenterologists have historically had limited input with these patients in the UK and the USA.
Accessing more information and patient support
Clinicians who are interested in obtaining more education or consulting guidelines about the management of NET patients are advised to consult the UKINETs (www.ukinets.org or Twitter @UKINETS) and ENETS (www.enets.org) websites for details, including training courses (eg, NETs for newcomers) and conferences. Excellent patient information and support in the UK is provided by Neuroendocrine Cancer UK (www.netpatientfoundation.org) and locally by NET centres and their specialist nurses. www.livingwithnets.com also provides helpful patient information.
Key points
The gastrointestinal tract and pancreas are the most common locations for neuroendocrine tumours (NETs), so these tumours are often first encountered by gastroenterologists.
There are frequently considerable delays in diagnosing NETs, as symptoms may be similar to those found in other commoner conditions such as irritable bowel syndrome.
Characterisation of a NET involves establishing a tumour’s grade, stage, primary site and secretory status and often requires specialist investigations.
Tumour resection is usually advised for most localised NETs.
Long acting somatostatin analogue injections represent the first line treatment option for most patients who have unresectable low grade NETs.
There are multiple treatment options for NETs especially in the second line setting and these should be discussed by a fully resourced NET multidisciplinary team (MDT).
Patient prognosis varies greatly, but can be excellent in some patients who have widespread metastatic disease.
Gastroenterologists play key roles in NET patient care from diagnosis to treatment and their involvement in a NET MDT improves the quality of patient-reported outcomes and experience.
Ethics statements
Patient consent for publication
References
Footnotes
Twitter @DrMohidKhan, @gastrolivuni
Contributors MSK and DMP both wrote sections of the first draft of this review and both revised the final article.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests DMP has received consultancy funding from Ipsen, Advanced Accelerator Applications and Mayoly Spindler laboratories and research funding to investigate gastric NETs from Trio Medicines. MSK has received consultancy and speaker fees from Ipsen,Advanced Accelerator Applications and Novartis.
Provenance and peer review Commissioned; externally peer reviewed.
Linked Articles
- UpFront