Article Text
Abstract
Patients with Crohn’s disease are at high risk of presenting with or developing a bowel stricture during the course of their disease. The available therapeutic options to manage a symptomatic Crohn’s stricture include medical therapy (mainly biologics), surgical resection and endoscopic interventions. The choice of therapeutic modality depends on the clinical presentation of the stricture, the nature of the stricture (inflammatory vs fibrotic, primary vs anastomotic) and its anatomical characteristics on endoscopy and imaging (length, number, location of strictures and severity of obstruction). The aim herein is to provide an overview of the comprehensive assessment of a Crohn’s stricture and to review the indications of the different therapeutic modalities, their success rates and their limitations to help clinicians properly evaluate and manage Crohn’s strictures.
- Crohn's disease
- endoscopic procedures
- IBD clinical
Statistics from Altmetric.com
Introduction
Crohn’s disease (CD) is a chronic relapsing inflammatory bowel disease (IBD) which is categorised into inflammatory, stricturing and penetrating phenotypes.1 Patients with purely inflammatory disease phenotype at diagnosis can exhibit inflammatory behaviour throughout their disease course or evolve over time to the stricturing or penetrating form of the disease.2 3 Almost 50% of patients with CD will develop strictures in their lifetime.4 5 Patients with strictures are at increased risk of developing internal penetrating disease (with fistula and abdominal abscess formation) and requiring CD-related surgery over time.6
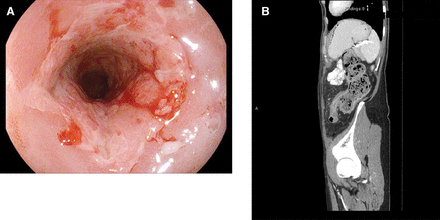
Strictures can occur anywhere in the gastrointestinal tract but commonly affect the terminal ileum. Strictures are classically classified into inflammatory and fibrotic. However, this classification is limited as most strictures have an overlap of inflammatory and fibrotic components (figure 1).4 CD severity, duration and small bowel involvement at the time of diagnosis, as well as smoking and CARD15/NOD2 gene mutation, are predictors of stricturing complications.3 7 History of stricture is another risk factor, with up to 46% of patients having stricture recurrence after surgical intervention.8 Strictures are also classified into primary and anastomotic. The incidence of anastomotic strictures (typically ileocolonic anastomosis stricture) is between 3% and 30% at a median of 5–12 months after colorectal surgery.9 10 Stricture recurrence is not dependent on the type of surgical anastomosis (handsewn vs stapled).9 11
(A) Mixed inflammatory and fibrotic sigmoid stricture and (B) CT scan showing circumferential wall thickening at the descending sigmoid colon junction measuring 4.5 cm in length associated with pericolonic fat stranding and fat creeping along the stricture, and the presence of mucosal hyperenhancement and wall oedema suggesting mixed inflammatory on top of the chronic fibrotic stricture.
The pathogenesis of strictures involves chronic inflammation and submucosal injury with exaggerated accumulation of extracellular matrix including collagen and smooth muscle cells, which ultimately results in fibrosis and stricture formation.4 This process occurs through inflammatory-dependent and inflammatory-independent mechanisms.4 This notion is supported by the little to no impact of anti-tumour necrosis factor (TNF) medications on the progression of strictures in patients with CD despite receiving therapy early after diagnosis.12 13
Patients with strictures often present to urgent care with acute obstructive symptoms of abdominal pain, distension and vomiting.4 However, chronic stricture can manifest with more insidious symptoms of low-grade abdominal discomfort, postprandial abdominal cramps and distension, borborygmi, and weight loss as patients often adopt a low-residue, low-volume diet to mitigate their gastrointestinal symptoms.14 Before proceeding with a surgical or endoscopic intervention on the stricture, it is important to assess whether the gastrointestinal symptoms are due to the stricture itself or to associated conditions such as small intestinal bacterial overgrowth, irritable bowel syndrome, lactose intolerance or coeliac disease. Of note, approximately 17% of small bowel Crohn’s strictures can be asymptomatic.15
Assessment of a Crohn’s stricture: colonoscopy and imaging
The diagnosis of Crohn’s stricture is first made on colonoscopy or imaging depending on the patient’s clinical presentation. The two diagnostic modalities are complementary and should be performed to properly assess the nature and the anatomical characteristics of the stricture and to guide therapy. Endoscopists usually grade the severity of strictures based on the passage of or inability to pass an adult colonoscope through the narrowed area with a reasonable pressure applied.16 When a stricture is suspected or previously seen on imaging, it is best to use a paediatric colonoscope or gastroscope to optimise the chances of traversing the stricture, or at least visualise the stricture well enough to determine its inflammatory and/or fibrotic nature, take biopsies and potentially treat it endoscopically. All strictures in patients with IBD should be biopsied to rule out the presence of dysplasia or malignancy (figure 2). A colonic stricture in ulcerative colitis is a malignancy until proven otherwise, and even though strictures are a known complication of CD they should always be biopsied, in particular if new or rapidly progressive. Taking into account the limitations of biopsying the edges of the stricture when it is not traversable, a high index of suspicion should be maintained in the right clinical context; based on a French retrospective study, 3.5% of patients with IBD were found to have dysplasia or cancer in colorectal strictures at the time of surgery, despite the lack of dysplasia on preoperative endoscopic biopsies.17 Completing the work-up with imaging can reveal a stricture-associated mass in case of malignancy. There is no described incidence in the literature of dysplasia or malignancy occurring in anastomotic strictures, and in our experience this risk is unlikely unless the initial intestinal resection was done for a dysplastic lesion.
(A, B) Stricture at the splenic flexure in a patient with Crohn’s disease which was biopsied and came back as adenocarcinoma.
CT enterography (CTE) and magnetic resonance enterography (MRE) are the standard modalities to assess strictures in patients with CD.18 In addition to stricture location, radiologists should document the degree of luminal narrowing, wall thickness and enhancement pattern, length of stricture, and the presence of prestenotic dilation on cross-sectional imaging.19 Inflammatory or fibrotic or mixed Crohn’s strictures are defined on cross-sectional imaging as a 25% increase in bowel wall thickness and a 50% reduction of the luminal diameter in comparison with the normal adjacent bowel loop.16 19 Hyperenhancement of the bowel wall on MRE after administration of gadolinium is the result of increased vascular permeability and angiogenesis and is seen in both inflammatory and fibrotic strictures. It is the pattern of enhancement that can help differentiate an inflammatory from a fibrotic stricture, with delayed hyperenhancement and layered wall enhancement seen in inflammatory strictures. Other findings on MRE that favour an inflammatory stricture include increased T2 mural signal intensity reflecting bowel oedema and increased mesenteric (vasa recta) vascularity, also known as the ‘comb sign’. Findings on MRE that are seen with a predominantly fibrotic stricture include low T2 mural signal intensity, mesenteric fat proliferation and prestenotic dilatation with a bowel wall diameter distended to more than 3 cm (table 1 and figure 3). Of note, fistulas can be associated with both inflammatory and fibrotic strictures.
MRE findings of inflammatory and fibrotic strictures
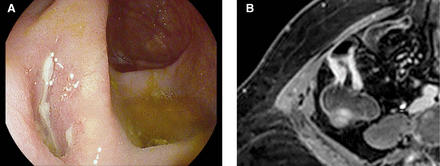
(A) Mainly fibrotic appearance of the ileocoecal anastomosis with smooth borders and one shallow ulcer, unable to pass the paediatric colonoscope; and (B) MRE showing thickened wall and hyperenhancement of the distal ileum over the ileocoecal anastomosis.
Hybrid positron emission tomography and MRE or CTE were shown to accurately differentiate between inflammatory and fibrotic strictures.20 Imaging features suggestive of a malignant stricture or the finding of an associated mass should prompt surgical evaluation.
Video capsule endoscopy (VCE) is often used for complete visualisation and assessment of the small bowel involvement in CD. However, this imaging technique is typically avoided when suspecting a small bowel stricture due to the potential risk of capsule retention. Patency capsule can be used first to provide direct evidence of functional patency of the gut lumen prior to VCE evaluation in patients with suspected stricture that is not visualised on radiological imaging.21
Intestinal ultrasound is a non-invasive imaging technique that has recently gained recognition as an accurate technique for assessment and monitoring of patients with IBD. It is an attractive alternative to CT/MRI for evaluation of bowel strictures for several reasons: there is no associated ionising radiation, no need for intravenous contrast and can be performed at the time of clinic visit, allowing for a management discussion with the patient in real time.22 23 In addition, real-time shear wave ultrasound elastography has the potential to differentiate between inflammatory and fibrotic strictures.24 These techniques can be particularly useful in the paediatric population as well as in patients whose body habitus and bowel anatomy allow for adequate ultrasound evaluation, preventing repeated lifetime radiation and intravenous contrast exposure from CT/MRI and avoiding the burden of oral contrast and the time spent in the radiology department.
Medical therapy of Crohn’s stricture
While no targeted antifibrotic therapies are available yet to prevent or treat Crohn’s stricture,25 patients with inflammatory or mixed inflammatory and fibrotic strictures commonly respond to anti-inflammatory medical therapy such as steroids in the acute setting and/or anti-TNF therapies for induction and maintenance of disease control.26 27 Effective medical therapy of inflammatory strictures can delay or prevent long-term complications.28 According to the CREOLE study, independent clinical and MRE predictors of successful response to induction and then maintenance with adalimumab in patients with a new diagnosis of small bowel Crohn’s stricture include recent (less than 5 weeks in duration) and severe (CD obstructive score >4) obstructive symptoms, concomitant use of thiopurines at the time of induction with adalimumab, total stricture length of less than 12 cm, maximal prestenotic small bowel dilation of 29 mm or less, absence of fistula, and marked enhancement on delayed phase.27 In this study, success at week 24 was defined as continuation of adalimumab, without the need for steroids, total parenteral nutrition, endoscopic balloon dilatation (EBD) or surgical resection of the stricture. The probability of success of medical therapy for Crohn’s strictures at week 24 was 89% in patients with a prognostic score of 4 or more and 61% in patients with a score of 3.
Additional considerations in the medical management of CD strictures include nutritional support to minimise gastrointestinal symptoms, but also provide patients with the calories and nutrients they need: a low-fibre diet or eating fibres in a more digestible form (smoothies, pureed) can decrease obstructive symptoms, while enteral nutrition with (semi)elemental diet can minimise inflammation, and high-protein oral nutrition and vitamin B12 and iron supplements when needed can optimise the nutrition status of patients (which are all equally important in medically managed patients or before the surgical resection of a stricture). In addition, smoking cessation counselling should be provided and psychological support offered to address the mental health needs of patients experiencing the complications of CD. These multidimensional interventions can help improve the clinical outcomes of patients with CD stricture.29–31
Endoscopic therapy intervention
Advances in therapeutic endoscopic techniques allow for non-invasive alternatives to surgery in the management of short fibrotic Crohn’s strictures and anastomotic strictures. We will review the indications and limitations of EBD, endoscopic stricturotomy (ESt), endoscopic stricturoplasty and self-expanding metal stents (SEMS).32 The long-term efficacy of endoscopic therapy in strictures is defined as surgery-free survival for 1 year after the procedure.
A few precautions need to be taken when considering an endoscopic therapy of a stricture: reduce corticosteroids dose before the procedure since steroid use is associated with a higher risk of procedure-associated perforation,24 use a paediatric colonoscope or a gastroscope if possible, use carbon dioxide insufflation,33 and consider having fluoroscopy available.
EBD is an effective intervention for fibrotic strictures of less than 5 cm in length without penetrating (fistula) complications and with a straight bowel lumen (non-angulated).34 35 EBD should be avoided in long strictures or strictures with deep ulcers: in strictures greater than 5 cm in length, the risk of complications from EBD and the need for surgery increase by 8% for every 1 cm increase in length36 and deep ulcers in strictures increase the risk of bleeding and perforation.37 EBD is performed using through-the-scope balloon which can be inflated to the desirable size (figure 4). Graded dilation is recommended to minimise the risk of bleeding and perforation,24 starting at a balloon size of 1–2 mm larger than the stricture diameter, holding the inflated balloon for 30–60 s, and then increasing the balloon size stepwise up to the next two sizes. The overall aim is to reach a balloon size of 5 mm above the initial stricture diameter and/or up to 18–20 mm balloon expansion. The risk of perforation increases with a balloon size of more than 25 mm.38 For tight strictures with a diameter of less than 10 mm, endoscopists can chose to dilate to smaller sizes, with repeat EBD sessions to achieve the desirable size. Multiple EBDs within a short period of time predict the need for surgical intervention.39 Technical and clinical success after EBD is defined as the passage of colonoscope through the previously non-traversable stricture with a reasonable pressure applied and relief of clinical obstructive symptoms, respectively. The risk of bleeding and perforation with endoscopic interventions should be discussed with the patient and that such complications could require additional interventions including urgent surgery. For these reasons, a therapeutic endoscopist and a colorectal surgeon need to be available should complications arise. EBD has a technical success of 89% and a clinical success rate of 81%, with a low overall risk of complications (<3%).36 However, up to 52% of patients will require repeat dilation and 30% will require surgery at 12-month follow-up after initial EBD. Hence, when considering EBD to manage a Crohn’s stricture, it is important to discuss with the patient that surgery or further dilatations at regular intervals might be needed to maintain or achieve durable symptomatic relief. Whether intrastricture steroid injection in addition to EBD has additional benefits on outcomes is controversial based on retrospective studies; however, the only prospective study in adults did not show any added benefit to steroid injection and there was actually a trend towards worse outcomes (need for redilation or surgery) in those who received steroid injection with EBD compared with EBD alone.40
(A) Ileocolonic anastomotic stricture; (B) endoscopic balloon dilatation using through-the-scope balloon across the anastomotic stricture; (C) ileocolonic anastomotic stricture post dilation; and (D) CT scan showing dilated small bowel with transition point at the ileocolonic anastomotic site where mild bowel thickening is noted.
In a meta-analysis by Hassan et al,41 anastomotic strictures were mainly managed by EBD. Interestingly, studies have shown no difference in the efficacy, outcomes (need for surgery or redilation) and safety of EBD, whether EBD was done for a primary CD stricture or for an anastomotic stricture.41 42
There is no clear guidance regarding the management of incidental strictures found on endoscopy in asymptomatic patients. Although dilation of asymptomatic strictures could delay the development of symptomatic strictures or the need for surgery, the decision to intervene should be a shared decision between the patient and the treating physician, weighing in the risks and benefits of such intervention.32
ESt involves needle knife electroincision or cauterisation of the stricture to break down the fibrous tissue and can be performed in a radial, horizontal or circumferential fashion.32 This technique is indicated for strictures refractory to EBD, angulated strictures and anorectal strictures, and has a lower recurrence rates of the stricture compared with EBD.43 The extent of ESt is largely empiric and subjective and mainly based on the endoscopist’s experience to determine the luminal patency that needs to be achieved after endotherapy.44 In one study, the risk of perforation with ESt was 2% and the risk of bleeding was 8%, whereas salvage surgery due to poor response to ESt was needed in 11% of patients.45
Endoscopic stricturoplasty, where endoscopic clips are applied after ESt, can enhance ESt efficacy and maintain luminal patency by widening and stabilising the incision site.32 ESt has a lower risk of perforation than EBD as it exerts targeted precise force to asymmetric strictures instead of the radial equal force of EBD. However, ESt has a higher risk of delayed bleeding that can be managed by rescue endoscopy to achieve haemostasis.
SEMS placement is another emerging treatment modality for Crohn’s stricture that appears to be highly effective and safe for short fibrotic stricture of the ileum and short ileocolonic anastomotic stricture.46 SEMS is a partially covered stent that can be removed endoscopically, and in a large single-centre series SEMS retrieval was performed 7 days after insertion for Crohn’s stricture. In this series of 21 patients, technical and clinical success was 100% and 80%, respectively, and no patient needed stricture-related surgery on follow-up period (range, 3–50 months).46 Asymptomatic stent migration occurred in three patients, but there was no stent-related impaction, perforation or bleeding. Future studies are needed to assess the overall efficacy and cost-effectiveness of SEMS compared with other endoscopic techniques, taking into account the higher cost of the stent and the cost of two endoscopic sessions (to place and remove the stent), as well as the potential cost savings from avoiding repeated intervention or surgery.47
Surgical intervention
Surgery, whether stricturoplasty or segmental resection of the diseased segment, is indicated in symptomatic predominantly fibrotic stricture, strictures associated with penetrating disease (fistula or intra-abdominal abscess) or significant prestenotic dilatation, and any stricture with atypical features suspicious for malignancy.48 It is also an effective and reasonable option for short fibrotic strictures or anastomotic stricture non-responsive to endoscopic therapies, as well as multiple small bowel strictures.32 49 The BACARDI study50 identified factors associated with a higher risk of surgery at 1 and 5 years after a Crohn’s stricture diagnosis and developed a risk stratification model to guide decision of a medical versus surgical approach. The five factors associated with a higher risk of surgery included prestenotic dilatation, associated perforating phenotype, prior or current anti-TNF exposure, CARD15/NOD2 mutation and a high C reactive protein (>11 mg/dL) at the time of stricture diagnosis. With each of these factors assigned a value of 1 (except for prestenotic dilatation which was assigned a value of 2), the following risk model and recommendations were developed. Surgery-free survival was nil at 1 year in patients with all risk factors (6 points) and only 50% and 20% at 1 and 5 years, respectively, in patients with 4–5 points; these patients benefit from early surgery after diagnosis of the stricture, instead of ineffectively cycling through different medical therapies. On the other hand, surgery-free survival was 80% at 5 years in patients with 0–1 point, identifying a low-risk group who would benefit from medical and/or endoscopic therapy. Patients with 2–3 points are at medium risk of surgery, with surgery-free survival around 80% at 1 year but dropping to 40% at 5 years; these patients benefit from optimising medical and/or endoscopic therapies and close monitoring and reassessment of the stricture every 6–12 months.
Conclusion
Strictures are a common complication of CD, and while it is crucial to rule out a malignant stricture most strictures are benign and often predominantly inflammatory or fibrotic in nature. Once a stricture is identified, it is important to accurately assess its impact on the patient’s symptoms, its nature and its anatomical characteristics on colonoscopy and imaging. The goals of CD stricture management are to relieve the patient’s obstructive symptoms and optimise preservation of bowel length and integrity.
The management of CD strictures should be individualised to the patient, taking into consideration several factors related to the patient (such as patient age, comorbidities and frailty, safety and efficacy of medical therapies, surgical risk), to the IBD phenotype and history (such as prior strictures resections, length of the remaining small bowel, perforating phenotype) and to the stricture itself (inflammatory or fibrotic, primary or anastomotic, length, number, location). The decision to proceed with medical, endoscopic or surgical therapy (or a combination of) is based on the clinical, endoscopic and radiological findings, as well as the local availability of the different therapeutic options and the patient’s preferences. Following an initial therapeutic intervention, clinical response should be assessed and the stricture should be monitored periodically for recurrence or progression and therapeutic modalities adjusted accordingly.
Overall, a multidisciplinary team that includes the IBD specialist, radiologist, therapeutic endoscopist, colorectal surgeon, nutritionist and psychologist is needed to determine the most appropriate therapeutic intervention for a CD stricture and to achieve the desired therapeutic outcomes.
Ethics statements
Patient consent for publication
Ethics approval
This study does not involve human participants.
References
Footnotes
Contributors MSI and AC reviewed the literature, drafted the manuscript and approved the final version. AC is the article guarantor.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests AC: consulting and/or advisory board for Pfizer, Janssen, Takeda, AbbVie and BMS.
Provenance and peer review Commissioned; externally peer reviewed.