Article Text
Abstract
Objectives The purpose of this trial was to evaluate the efficacy and safety of recently developed suppositories containing free fatty acids as a bowel-cleansing agent prior to flexible sigmoidoscopy and compare them with Klyx (docusate sodium/sorbitol).
Design A controlled, non-inferiority, single-blind, randomised study on outpatients undergoing flexible sigmoidoscopy.
Setting Department of Gastroenterology, Landspitali-University Hospital and endoscopic clinic.
Patients 53 outpatients undergoing flexible sigmoidoscopy.
Intervention Participants were randomised to receive either free fatty acid suppositories (28) or a standard bowel preparation with Klyx enema (25). In the study group, two suppositories were administered the evening before as well as 2 h prior to the sigmoidoscopy. In the control group, Klyx enema (120 mL) was administered the evening before and repeated 2 h prior to the procedure.
Main outcome measurements Quality of the bowel cleansing, height of scope insertion and safety.
Results The mean height of scope insertion and bowel cleansing was 43 cm (SD=13.4) in the study group and 48 cm (SD=10.4) in the control group (NS). The investigating physicians were less satisfied with the bowel preparation in the study group compared with the control group with a difference of 20% (p<0.016). The amount of faeces noted in the rectum was similar in both groups with no significant difference (p<0.56). No serious side effects, toxic reaction or irritation were observed.
Conclusions The suppositories are well tolerated with no significant side effects. The suppositories had distinct bowel emptying effect and as effective as Klyx in rectal cleansing. Although physician's satisfaction was slightly lower, the height of scope insertion was similar.
Trial registration number EudraCT nr.: 2010-018761-35.
- CLINICAL TRIALS
- COLONOSCOPY
- ENDOSCOPY
- LIPIDS
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Flexible sigmoidoscopy is a routine procedure performed by gastroenterologists and many surgeons for the diagnosis, control and treatment of patients with left-sided colorectal symptoms.1 Bowel preparation for flexible sigmoidoscopy should provide a clear view of the intestinal lumen without causing irritation or inflammation in the mucosa. The visualisation of the mucosa must always be the most important factor especially concerning the evaluation of potential malignant changes. Non-acceptance of suboptimal bowel preparation is also required in order to ensure universally high standards in screening procedures. The cleansing should be easy for the patient to administrate and take effect soon after administration.2–4
Several preparations for bowel cleansing for flexible sigmoidoscopy are available today, and the method of bowel cleansing varies between countries and individual clinics.5 The optimal and most cost-effective bowel-cleansing regimen is not known.5 Only a few studies have compared the different bowel-cleansing methods for flexible sigmoidoscopy.6 Common methods of bowel cleansing include enemas, sulfate-free polyethylene glycol electrolyte solution, bisacodyl, senna, cascara, castor oil, magnesium citrate and oral sodium phosphate.7 According to the guidelines from the American Society for Gastrointestinal Endoscopy, one or two enemas are recommended as the bowel preparation of choice for flexible sigmoidoscopy.7
Recently, suppositories containing free fatty acids derived from cod liver oil, as the active ingredient, were studied in a randomised, double-blind, placebo-controlled clinical trial.8 In this trial, these suppositories were found to have a clear laxative effect compared with placebo, where 90% in the study group defecated compared with 33% in the control group.8 This study also showed that the suppositories were safe to use with no significant difference in side effect between the two groups.8
The purpose of this trial was to evaluate the efficacy and safety of these recently developed suppositories as a bowel-cleansing agent prior to flexible sigmoidoscopy and compare them with Klyx (docusate sodium/sorbitol), which is the standard preparation used for cleansing prior to flexible sigmoidoscopy in our institutions.
Methods
This was a controlled, non-inferiority, single-blind randomised prospective clinical trial on outpatients undergoing flexible sigmoidoscopy in three study centres. This trial is registered at ClinicalTrials.gov (EudraCT nr.: 2010-018761-35). This study was approved by the National Bioethics Committee of Iceland, the Icelandic Medicines Agency and the Data Protection Authority.
All patients referred to our institutions for flexible sigmoidoscopy during the period of November 2010 to November 2011 were invited to participate in the study. After a screening visit, all eligible candidates, after signing an informed consent, were enrolled in the study and randomised to either the study group or the control group by block randomisation. The inclusion criteria included patients with previous history of rectal bleeding, follow-up examination after rectosigmoid surgery or for polyps. Patients under the age of 18, pregnant women and patients with diarrhoea or active bleeding from rectum at the time of the examination as well as all patients receiving any laxative treatment were excluded. A total of 53 patients participated in the study. The number of subjects included and their demographics are shown in table 1. One of the participants in the Klyx group did not answer the patient's questionnaire. The most common indication for the flexible sigmoidoscopy was bleeding per rectum (table 1). Patients were randomised to receive either the study suppositories or a standard treatment with self-administered Klyx enema (Ferring Pharmaceuticals, Switzerland). In the study group, the participants self-administered two suppositories, the evening before, as well as the following morning, prior to the flexible sigmoidoscopy. In the control group, Klyx enema (120 mL) was, in a similar manner, self-administered the evening before the procedure, as well as the following morning, prior to the procedure. Before undertaking the sigmoidoscopy, participants filled out a questionnaire about the possible side effects of the cleansing, that is, if there was any irritation or bleeding, if and when they felt the urge for defecation, if and when they defecated, as well as if the smell of the preparations (suppositories and Klyx) during the treatment was bothering. The investigating physicians were masked for which bowel cleansing the participant received and, because of a potential revealing smell from the suppositories, they wore a surgical mask containing perfume during the sigmoidoscopy. The physicians filled out a questionnaire about the amount of faeces noted in the rectum and sigmoid colon, any bleeding or mucus, total view, how satisfied the investigating physicians were with the bowel preparation, the depth of scope insertion, as well as whether they felt that the endoscopy should be repeated. The endoscopists were all experienced and certified specialists in gastroenterology and endoscopy.
Demographics and indications for the examinations
Statistical analysis
Statistical tests were performed with the statistical software R, V.2.15.3. Difference in means was tested with a Welch two-sample t test. χ2 test for homogeneity was used to compare the results for Klyx and the suppositories for categorical variables. Difference in medians was tested with Wilcoxon rank sum test. All reported p values were two-tailed. The level of significance was set at 0.05.
Results
After the first application of the suppositories, 12 out of 28 participants (43%) felt the urge to defecate within 30 min. Subsequently, 7/28 (25%) had bowel movements within 30 min. In the group using Klyx, 23/24 (96%) felt the urge to defecate within 30 min. Subsequently, 22/24 (92%) had bowel movements (table 2). The proportion of individuals that felt the urge to defecate within 30 min was higher for the group using Klyx (p<0.001) (table 2). The proportion of individuals that had bowel movements within 30 min was higher for the group using Klyx (p<0.001). After the second application of the suppositories, 13/28 (46%) felt the urge to defecate within 30 min. Subsequently, 11/28 (39%) had bowel movements within 30 min. In the group using Klyx, 24/24 (100%) felt the urge to defecate. Subsequently, 24/24 (100%) had bowel movements within 30 min. Five subjects (18%) did not have any bowel movement after using the suppositories twice, which gives a total efficacy of 82% in the study group (table 2). The proportion of individuals that felt the urge to defecate within 30 min was higher for the group using Klyx (p<0.001). The proportion of individuals that had bowel movements within 30 min was higher for the group using Klyx (p<0.001). Complaints of pruritus, pain or blood per rectum were not significantly different in the two groups; however, the smell was significantly worse in the study group (data not shown).
Time until the urge for bowel movement and time until bowel movement after administration of suppositories or Klyx
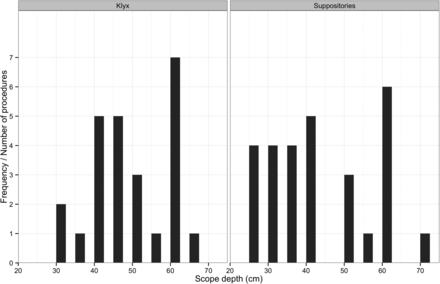
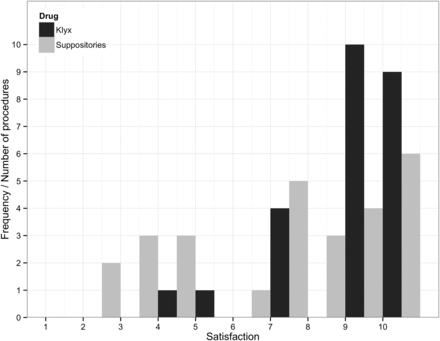
The mean depth of scope insertion was 43 cm (SD=13.4) in the study group and 48 cm (10.4) in the Klyx group. The difference was non-significant (figure 1). The amount of faeces noted in the rectum was similar in both groups with no significant difference (table 3). However, larger amounts of faeces were noted in the sigmoid colon in the study group (table 3). Mucus was noted in 9/28 (32%) subjects in the study group compared with 15/25 (60%) subjects in the Klyx group. The amount of blood observed was similar in both groups (table 3). The overall view rated by the physicians as good, average or bad was higher in the Klyx group, although the differences were small (figure 2). Rated on a scale from 1 to 10, the investigating physicians were less satisfied with the bowel preparation in the study group (median 7) compared with the Klyx group (median 9) (figure 3). No physician felt the need to repeat the endoscopy.
Frequency of depth of scope insertion in centimetre for Klyx (on the left) and suppositories (on the right) (p=0.09).
The amount of faeces noted in the sigmoid colon and rectum as well as the amount of blood and mucus noted during the endoscopy
The overall endoscopic view as rated by the physician (p=0.054).
Physicians’ satisfaction with bowel cleansing (p≤0.016).
No serious side effects, toxic reaction or irritation were observed in either group.
Discussion
The suppositories were well tolerated with no significant side effects. The suppositories are as good as Klyx in regards to providing view of the rectum but were inferior to Klyx in bowel preparation according to physicians’ satisfaction, giving less total view because of more faeces in the sigmoid colon.
The investigating physicians were significantly less satisfied with the bowel preparation in the study group. However, according to their assessment, there was not a need to repeat the examination because of poor bowel preparation. In a recent study, approximately 8% of examinations had to be repeated because of poor bowel preparation.1
Studies investigating the optimal form of bowel preparation for flexible sigmoidoscopy are lacking,9 ,10 and no gold standard exists.1 ,3 The only available guidelines from the American Society for Gastrointestinal Endoscopy are from 1988.7 The studies on bowel preparation for flexible sigmoidoscopy so far have compared many types of bowel preparations, both different types of enemas, enemas vs. per oral preparations, combination of per oral treatment and enemas, as well as suppositories vs. enemas. Most studies have a focus on the quality of the preparation concerning the endoscopic view6 ,5 ,9–14 while others focus on the variability in adenoma detection rates.2 ,9 Although enemas seem to be the preferred by most physicians,9 there are several options in bowel preparation for patients undergoing flexible sigmoidoscopy, both regarding type of medication and administration route and the timing of the administration. Oral preparations have, in some studies, shown to be superior to enemas,6 ,9 ,10 whereas other studies have found enemas to be superior to oral preparations.11 ,12 However, other researchers have not found any differences between enemas and oral preparations.13 ,14 Few studies have compared suppositories with other form of cleansing, thus making it somewhat difficult to compare our results with the results of previous studies. Underwood et al conducted a study on 203 patients undertaking flexible sigmoidoscopy who were randomised to receive one Fleet ready-to-use enema (sodium dihydrogen phosphate dihydrate, disodium phosphate dodecahydrate) or 2×4 g glycerin suppositories 2 h prior to the procedure. They found that the average depth of endoscope insertion in the enema group was significantly deeper compared with the group receiving suppositories. Moreover, the physicians grated the quality of preparation as excellent in 67% among the patients in the enema group as compared with only 17% in the glycerin group.15 These results are in line with our findings, although the difference between the suppositories and enema was less evident in our study.
The quality of bowel preparation scale (excellent, good, adequate or poor) derived from the Walter Reed Army Medical Center10 or the assessment used in the United Kingdom flexible sigmoidoscopy trial (UKFSST) study16 do not distinguish between the amount of faeces in the rectum and in the sigmoid. It is important to note this difference as cleansing for flexible sigmoidoscopy should provide the same view in the sigmoid colon as in the rectum. In this study, the amount of faeces noted in the sigmoid colon was significantly higher in the study group, but the amount of faeces in the rectum was small and similar in both groups.
Although the bowel preparation for the flexible sigmoidoscopy was better with Klyx, it is possible that the suppositories are more appropriate as preparation for proctoscopies. This as well as the effect of dose escalation remains to be studied.
Conclusion
The suppositories were well tolerated with no significant side effects. The suppositories are as good as Klyx in regards to providing view of the rectum. The optimal use of these suppositories could be for patients undergoing proctoscopy and to initiate rectal evacuation when needed. The laxative effect of the suppositories is confirmed, as is their safety.
Significance of this study
What is already known about this subject?
The newly developed study suppositories were shown to lead to rectal evacuation in previous trial.
There are few clinical trials published on bowel preparation with suppositories and the role of suppositories in bowel cleansing is not fully investigated.
The optimal and most cost-effective bowel cleansing regimen is not known.
What are the new findings?
The suppositories are well tolerated with no significant side effects.
The suppositories had bowel emptying effect.
The suppositories were not inferior to Klyx in terms of depth of scope insertion and rectal cleansing.
How might it impact on clinical practice in the foreseeable future?
Suppositories for bowel cleansing could have greater role in the future.
The optimal use of the study suppositories could be in the preparation of patients undergoing rectoscopy and to initiate rectal evacuation when needed.
Acknowledgments
The authors thank Lysi Ltd. for helping with the development of the lipid suppositories and Gattefossé for donating ingredients. We also thank the nursing staff, especially Herdis Astradsdottir and Thora Thrainsdottir, for their contribution to the study.
References
Footnotes
Contributors All authors agree to be accountable for all aspects of the work. All authors contributed to the work in accordance with the following criteria. Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. OTO: main author. GMA: data analysis and interpretation of data. ES: contributed to the writing as well as revising of the data. TL: contributed to the writing and revising of the article. JOK: main study physician contributions to the design of the work. SHL: statistical analysis. ESB: contributed to the writing and final approval of the version to be published.
Funding This study was funded by the Technology Development Fund of Iceland (official fund, $40 000) with no interest in the product.
Competing interests OTO has served as a speaker, a consultant and an advisor for Lipid Pharmaceuticals. GMA is an employee of Lipid Pharmaceuticals. ES and TL own shares in Lipid Pharmaceuticals.
Patient consent Obtained.
Ethics approval National Bioethics Committee of Iceland, the Icelandic Medicines Agency and the Icelandic Data Protection Authority.
Provenance and peer review Not commissioned; externally peer reviewed.