Article Text
Abstract
Lynch syndrome (LS) is a dominantly inherited cancer susceptibility syndrome defined by presence of pathogenic variants in DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2, or in deletions of the EPCAM gene. Although LS is present in about 1 in 400 people in the UK, it estimated that only 5% of people with this condition are aware of the diagnosis. Therefore, testing for LS in all new diagnoses of colorectal or endometrial cancers is now recommended in the UK, and gastroenterologists can offer ‘mainstreamed’ genetic testing for LS to patients with cancer. Because LS results in a high lifetime risk of colorectal, endometrial, gastric, ovarian, hepatobiliary, brain and other cancers, the lifelong care of affected individuals and their families requires a coordinated multidisciplinary approach. Interventions such as high-quality 2-yearly colonoscopy, prophylactic gynaecological surgery, and aspirin are proven to prevent and facilitate early diagnosis and prevention of cancers in this population, and improve patient outcomes. Recently, an appreciation of the mechanism of carcinogenesis in LS-associated cancers has contributed to the development of novel therapeutic and diagnostic approaches, with a gene-specific approach to disease management, with potential cancer-preventing vaccines in development. An adaptive approach to surgical or oncological management of LS-related cancers may be considered, including an important role for novel checkpoint inhibitor immunotherapy in locally advanced or metastatic disease. Therefore, a personalised approach to lifelong gene-specific management for people with LS provides many opportunities for cancer prevention and treatment which we outline in this review.
- CANCER GENETICS
- CANCER SYNDROMES
- COLONOSCOPY
- ASPIRIN
- SURGICAL ONCOLOGY
Statistics from Altmetric.com
What is already known about Lynch syndrome?
Lynch syndrome (LS) pathogenic variant carriers have an increased risk of a broad range of predominantly epithelial cancers, most frequently colorectal and endometrial cancer.
All new diagnoses of colorectal or endometrial cancer should be assessed for potential LS with mismatch repair (MMR) testing, and where defective, should trigger referral for constitutional MMR gene testing.
Gastroenterologists and other non-genetics service clinicians may now offer ‘mainstreamed’ genetic testing for LS to patients with cancer.
Patients diagnosed with LS require lifelong coordinated multidisciplinary care, where the application of interventions provides many opportunities to reduce cancer risk.
What surveillance should these patients undergo?
Colonoscopic surveillance should be performed every 2 years starting at age 25 years for MLH1, or MSH2 pathogenic variant carriers, or age 35 years for MSH6, or PMS2 pathogenic variant carriers.
Endoscopic lesions can be difficult to recognise due to a high frequency of flat non-polypoid morphology, and high-quality colonoscopy is essential.
Gynaecological surveillance has no proven benefit.
Aspirin reduces long-term colorectal cancer (CRC) risk by approximately 50%. Recommended doses include 150 mg ODonce daily or 300 mg ODonce daily for patients with BMIbody mass index >30.
What surgical treatments are recommended?
Women should be counselled on prophylactic hysterectomy and bilateral salpingo-oopherectomy from age 40 years (MLH1, MSH2 and MSH6 variant carriers).
There is a gene-specific approach to surgical management of CRC which takes in to account other patient factors.
What systemic oncological treatments are recommended?
Chemoprophylaxis with daily aspirin for at least 2 years is recommended in patients <70 years old diagnosed with LS to reduce long-term CRC risk.
Personalised systemic anticancer therapy is feasible for locally advanced or metastatic disease associated with LS, and may respond very well to relatively novel checkpoint inhibition immunotherapy.
Introduction
Lynch syndrome (LS) is an autosomal dominant inherited condition defined by the presence of a constitutional pathogenic variant in one of the mismatch repair (MMR) genes MLH1, MSH2, MSH6 and PMS2, or in deletions of the EPCAM gene which regulates gene MSH2 expression. LS is a multisystemic cancer predisposition syndrome which increases the lifetime risk of colorectal cancer (CRC), upper gastrointestinal (GI) tract malignancy, urinary tract, skin and others in both men and women, and endometrial and ovarian cancer in women and prostate cancer in men. These LS-related cancer diagnoses are common in younger people below of national screening ages, with a significant impact on quality-adjusted life years gained through LS diagnosis and prevention. There are a range of interventions which significantly improve outcomes in this population, either through prevention or early diagnosis of cancer, and these include regular colonoscopy, chemoprophylaxis and preventative gynaecological surgery (figure 1). Due to the increased risk of CRC, patients with LS are offered enrolment into a 2-yearly colonoscopic surveillance programme designed to improve prevention and early diagnosis of CRC.
Summary of recommended clinical interventions in people diagnosed with LS, with a gene-specific approach to colonoscopic, gynaecological or surgical management. LS, Lynch syndrome; PGD, pre-implantation genetic diagnosis; TAH-BSO, total abdominal hysterectomy and bilateral salpingo-oopherectomy.
LS is the most common form of hereditary colon cancer. It accounts for 3.5% of all CRC and endometrial cancer cases. Within the UK, LS is known to affect up to 1 in 450 persons.1 It is predicted that 175–200 000 patients in the UK have this condition. The point prevalence of cancer in the adult Lynch population is 4%–5%,2 therefore above the 3% threshold in symptomatic general population used to determine referral pathways for 2-week wait rules in the UK.3
There is gene-specific lifetime risk of cancer development depending on the underlying MMR gene pathogenic variant. For example, the lifetime risk of CRC is highest in patients with MLH1 variant 44%–53%, with some studies reporting up to 70%, MSH2 42%–46%, MSH6 18%–20% and lowest in carriers of PMS2 variant around 10%–13%.4–6 Consequently, clinical management varies depending on this gene-specific risk.
Diagnosis and recognition of LS
LS is defined by the presence of constitutional pathogenic variants (what were previously called inherited mutations) in MMR genes: MLH1, MSH2, MSH6, PMS2, as well as EPCAM. Previously, only some selected patients who had a diagnosis of CRC were offered testing for LS7–9; however, since 2017, ‘universal’ testing of all new CRC diagnoses is recommended by the National Institute of Health and Care Excellence (NICE).10 All CRCs should undergo testing for mismatch repair deficiency (dMMR) testing on their tumour specimen, preferably on colonoscopic biopsies where possible.
The presence of dMMR indicates faulty DNA replication base pair ‘mismatches’, which have not been repaired. Loss of protein expression of MMR proteins on immunohistochemistry (IHC) testing of cancers may indicate that the underlying gene requires testing. Alternatively, microsatellite instability (MSI) is a PCR-based test which identifies features of dMMR in repetitive DNA segments called microsatellites (which are susceptible to insertion–deletion mutations in tumours). Either IHC or MSI of tumour specimens may be performed as an index test in patients with CRC to identify those who may benefit from further assessment for LS (figure 2). IHC detects over 90% of cases of LS.11 In addition, MMR-deficient tumours may be susceptible to relatively novel checkpoint inhibitor immunotherapy, and have recently been approved in routine clinical practice.12 Subsequently, tumours with loss of MLH1 expression of MSI may require a further test to detect MLH1 promotor methylation, or may be immediately eligible for genetic testing of IHC is abnormal for proteins other than MLH1. If a tumour is dMMR with either abnormal IHC, or MSI, and no evidence of MLH1 promotor methylation, the patient is eligible for genetic testing for LS. Of note, any patient diagnosed with CRC under the age of 40 years may be offered germline testing directly.
Genomic medical service alliance Lynch syndrome (LS) project guidance: recommendation for testing following from IHC in new diagnosis of CRC (with thanks to Laura Monje-Garcia and Nicholas West). CRC, colorectal cancer; GLH, Genomic Laboratory Hub; IHC, immunohistochemistry; MMR, mismatch repair; MSI, microsatellite instability; MDM: Multidisciplinary Meeting.
The optimal pathway is that genomic testing be offered locally by the gastroenterologist, or other CRC multidisciplinary team (MDT) member, a process called mainstreaming, but patients may alternatively be referred to a clinical genetics team. Currently, there is an National Health Service (NHS) programme designed to deliver effective testing for LS delivered by cancer team clinicians locally.13
People with LS may also be diagnosed by a process of cascade testing of relatives of known individuals in a family, or because of a family history of cancer for example.
Congenital dMMR is a recessive form where LS is inherited from both parents, and is associated with a childhood malignancy spectrum which encompasses glioblastoma multiforme, haematological and GI malignancies; as an ultra-rare disorder, this should be managed in an expert centre as per British Society of Gastroenterology (BSG) guidelines.13
Lifelong management of LS
Colorectal surveillance
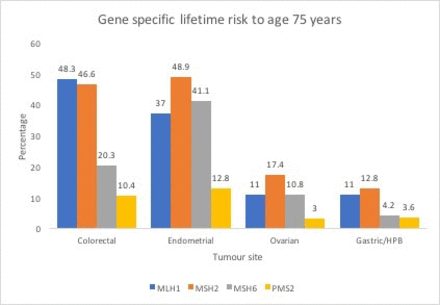
The cumulative lifetime incidence of CRC in people with LS (MLH1 45%, MSH2 35%, MSH6 20% PMS2 14%) is considerably higher than in the general population.5 Patients with LS have an accelerated pathway to carcinogenesis compared with the general population.14 Seminal work suggested that colonoscopy more than halves the risk of CRC, preventing CRC deaths as well as decreasing overall mortality by about 65% in LS families.15 As such, colorectal surveillance with routine colonoscopy every 2 years should start at 25 years for MLH1 and MSH2 carriers or at 35 years for MSH6 and PMS2 gene carriers.13 16 17 Historically, MLH1 and MSH2 carriers have been over-represented due to their stronger phenotype; however, there are now more patients with other genotypes being diagnosed with cancer11 (gene-specific cancer risk: figure 3 18).
Gene-specific lifetime risk of colorectal, endometrial, ovarian and gastric/hepatobiliary (HPB) cancers.
Patient experience is especially important in this high-risk population who may be expected to have 2-yearly colonoscopy from age 25 years onwards.19 Newton et al 20 demonstrated that different hospital recall systems, along with varying clinician and patient issues, resulted in very variable compliance with the recommended surveillance intervals for LS.
Despite adherence to appropriate, good-quality endoscopic surveillance, there are still high rates of interval CRC in LS21–23 (figure 4). This population may have distinct carcinogenesis pathways, often without a well-defined polyp stage, with a high prevalence of proximal neoplasia with flat morphology. There is an hypothesis that LS CRCs may arise from subepithelial endoscopically so-called ‘invisible’ lesions14 24; however, the true incidence of this phenomenon is unknown and remains controversial. A high proportion of post-colonoscopy cancers in LS are caused by missed lesions as a result of inadequate examination, lack of adherence to surveillance recommendation and incomplete polyp resection.25 Reassuringly, a large multicentre study recently showed that the rate of post-colonoscopy CRC in centres complying with current guidance was 1.2%.23 Colonoscopy is not only important in prevention of CRC but also in early diagnosis and therefore earlier stage CRC.
Surveillance-detected colorectal cancers from MSH6 (top left) and MSH2 carriers (top right white light (WLI), bottom left submucosal injection pre-cold snare polypectomy, bottom right MLH1 descending colon adenocarcinoma).
We believe it is especially important that colonoscopy in this population is performed to a high standard by a high-performing colonoscopist, with a well-prepared bowel, and emphasis on a good patient experience. Although randomised controlled trial data do not support the use of dye-spray extubation,26 we consider it good practice to slowly extubate, over approximately 15 min, with double pass intubation, and the use of assistive devices such as the Endocuff. These and other adjuncts may assist with identification of subtle neoplasia with flat morphology, which make detection challenging, although trials of technologies such as artificial intelligence (AI) may advance the practice further.23 27
In 2016, a multisociety meeting recommended the development of a quality-assured surveillance programme for people with LS,28 and from April 2023, the national screening programme in England will deliver this surveillance. This new programme will ensure adequate call and recall for people with LS and address the importance of high-quality colonoscopy. It is estimated that approximately 40 colonoscopies will be required per screening centre per year to deliver LS screening with an anticipated 10% increase year on year.
Upper GI surveillance
The gene-specific risk of developing upper GI malignancy ranges from <1% to 13%, but is highest in MLH1 and MSH2 gene carriers.13 16 17 The role of surveillance with upper GI endoscopy is not proven to alter stage of diagnosis or survival, however in theory it may identify precancerous lesions such as gastric atrophy or intestinal metaplasia or duodenal adenomas.29 However, further work is required to appreciate the potential benefits of upper GI endoscopy in this population.
Routine Helicobacter pylori eradication may benefit persons with a family history of gastric cancer in a first-degree relative.30 Many of the precursors identified at endoscopy are manifestations of chronic H. pylori infestation, and therefore it may be the case that management of H. pylori is a key risk-modifying intervention in people with LS.
Gynaecological surveillance and risk management
Lifetime risk of endometrial cancer is between 20% and 70% for MLH1, MSH2, MSH6 variant carriers, and 10% and 15% for PMS2 variant carriers, with a lesser but nevertheless increased risk of ovarian cancer. However, endometrial or ovarian cancer surveillance does not reduce mortality in LS.31 32 The typical age of onset of gynaecological cancer in LS is after age 40 years (with 5% patients younger than 40 years); therefore, women should be counselled on consideration of risk-reducing hysterectomy and removal of ovaries at age 40 years (for MLH1, MSH2 and MSH6 variant carriers). Women with LS should be counselled about red flag symptoms for endometrial and ovarian cancer such as abdominal distension, non-menstruation-related bleeding, urgency of urination or pelvic/abdominal pain in order to prompt early intervention and diagnosis.
Pre-implantation genetic diagnosis of embryos can be offered within NHS to all patients with LS, providing the family planning option of having a child who has not inherited the disease.
Medical interventions
Aspirin
Meta-analyses by Rothwell et al assessing observational data among populations taking aspirin demonstrated an absolute risk reduction of 20% in all cancers; however, within GI cancers, the benefit was as much as 34% risk reduction, which was statistically significant. Notably, there is typically a ‘lag-period’ of approximately 7–8 years after commencing aspirin before a difference in CRC risk manifests in people taking aspirin compared with a population not taking aspirin. These studies had important implications for the understanding of carcinogenesis and mechanisms of therapy to manipulate CRC risk,33 34 and contributed to the development of chemoprophylaxis studies including the Colorectal Adenoma/Carcinoma Prevention Programme (CAPP).
CAPP1 did not demonstrate the efficacy of aspirin in patients with polyposis; however, trials in people with LS were more promising. The CAPP2 trial recruited people with LS aged 45 years and over-randomising a total of 861 patients. These patients were randomised to receive either 600 mg of aspirin or placebo. Although the primary outcome of a difference in CRC incidence at 5 years was not confirmed, cancer outcomes at a mean of 10 years showed that 9% (40 of 427) in aspirin group developed CRC and 13% (58 of 434) in placebo group, which was statistically significant.35 Adverse events were similar across the two groups as were development of non-colorectal LS-associated cancers.
In 2019, the BSG guidelines recommended that aspirin be offered to people with LS,13 and in 2020, NICE made the same recommendation, producing a decision aid36 to help patients understand the benefits of aspirin in the context of a diagnosis of LS. We consider this decision aid a useful document which may be shared with patients as part of their clinical consultation.36
Although the frequency of adverse events was not higher in the aspirin versus placebo arm of the CAPP2 Study, we suggest some caution in specific clinical circumstances. We recommend that general practitioners check H. pylori status and treat accordingly, both as this may potentially reduce the risk of upper GI side effects with aspirin, but also may have an impact on gastric cancer risk in a population who are at higher risk.
Further data have been published which inform this decision-making since the publication of UK guidelines. We would advise that patients currently undergoing cancer treatment wait until this treatment has completed before commencing aspirin. The ASPREE trial demonstrated an increase of cancer diagnoses and adverse effect on later stages of cancer evolution in a trial of patients starting aspirin aged over 70 years. Although it is unclear, this observation may be due to aspirin suppressing the inflammatory response and facilitating metastasis.37 Therefore, we suggest patients diagnosed with LS aged 70 years or over should not start preventative aspirin.
The CAPP3, a non-inferiority study (in progress), compares different doses of aspirin,35 and is due to report first results in 2024. Pending these data, we recommend 150 mg daily upon diagnosis of LS, with a dose of 300 mg daily for those with a raised body mass index, because the efficacy of aspirin is reduced in obese patients.38 It is important to consider the ‘lag period’ for efficacy of aspirin means that the benefit will accrue from around 7 years after aspirin is commenced. In addition, data from CAPP2 suggest that aspirin should be taken daily for at least 2 years, and up to 5 years in total, after which it may be discontinued.
A personalised approach to cancer treatment
Surgical resection of CRC
The decision regarding surgery should be a patient-centred, multidisciplinary approach, taking into account patient wishes, gene-specific risk, comorbidities and age. For a patient with a pathogenic variant in MLH1 or MSH2, extended surgery may be considered to reduce risk of metachronous CRC; however, for patients with a pathogenic variant of MSH6 or PMS2, standard segmental colonic resection should be offered.17 In meta-analysis of metachronous CRC risk in LS, those who underwent extensive colectomy had an absolute risk of metachronous CRC of 4.7% (270 patients) vs 22.4% (1119 patients) in those who underwent segmental colectomies.39
Systemic anticancer therapy
In early-stage CRC, having dMMR is considered a positive prognostic indicator compared with late stage. In stage II CRC, there is considered to be a small margin of benefit in all comers (both dMMR and MMR proficient (pMMR)) and treatment with adjuvant chemotherapy is usually reserved to high-risk patients.40 The prognosis of dMMR over pMMR early-stage tumours is favourable.41–43 Therefore, MMR/MSI status should be used to identify patients with stage II disease who are at low risk of recurrence, and adjuvant therapy should not be routinely recommended.44
The advent of checkpoint inhibitor immunotherapy is a significant recent advance in dMMR CRC as well as across a range of dMMR cancers (figure 5). The KEYNOTE clinical trials, specifically KEYNOTE-028, showed an overall response rate (ORR) of 40% in patients with dMMR as well as a disease control rate (DCR) of >12 weeks in 90% of patients compared with ORR 0% in pMMR patients and DCR >12 weeks in 11% of patients.45 This study led to accelerated Food and Drug Administration (FDA) approval of pembrolizumab for patients with advanced CRC, MSI-High (MSI-H) or dMMR malignancy progressing through conventional therapy, and pembrolizumab is now NICE approved for first-line treatment of advanced CRC in the UK with dMMR/MSI.12
Complete clinical response in a patient who received second-line checkpoint inhibitor immunotherapy, after failure of response to standard fist-line chemotherapy.
How to implement an LS service
Implementation of high-quality family cancer service can improve appropriateness of colonoscopy, adenoma detection rate and tumour MMR testing.26 Optimal LS lifelong management requires support for patients and their families locally, with expertise provided by an MDT which includes gastroenterology, surgery, oncology, specialist nursing care and others affiliated to regional genetics services. A survey of CRC MDTs in the UK identified wide variability in pathways for patients with hereditary cancer, and lack of adherence to national guidelines46; however, in February 2022, NICE quality standards recommended that a lead clinician be identified in each cancer MDT to ensure delivery of testing for LS following a CRC diagnosis.47 Within the NHS, ‘mainstreaming’ by clinicians from cancer teams is being supported in order to optimise the streamlined and effective diagnosis of LS in patients with cancer. LS champions within each cancer team are being identified. Data from the National Disease Registration service demonstrated that in 2019, despite NICE guidance, only 42% of patients diagnosed with colon or endometrial cancer underwent MMR testing. Barriers to service improvement included work constraints and burnout related to the COVID-19 pandemic, but engagement with service improvement will significantly facilitate systematic delivery of universal LS testing.
There is evidence from registry-based studies that enrolment in screening via registries reduced CRC mortality.48 The benefits of registration allow streamlined organisation of families, tissue and information dissemination. From a patient-focused perspective, this allows for continuity of care, access to genetic counselling and testing and involvement in clinic trials.28 From a research perspective, this will facilitate epidemiological and molecular genetic studies, biobank of blood/tissue and prevention and therapeutics.49
Future areas of research
Development of LS vaccine
LS-associated cancers, when they develop, have a high somatic mutation rate. This makes them hypermutated and they generate frameshift peptides which are highly neoantigenic. Neoantigens can trigger a strong immunogenic anti-tumour response and there are data to suggest specific neoantigens that are present in LS across different patients. These neoantigens and subsequent recognition of immune reaction, via tumour infiltration and peripheral T cells, have been recognised to these neoantigens. This has led to development of an LS vaccine.50 This has shown benefit in mouse models supporting clinical benefit of recurrent neoantigen vaccination and is in early phase clinical trials in the USA and Germany.51
Faecal immunohistochemical testing for Lynch study
Management of hereditary CRC guideline publication preceded the COVID-19 pandemic, meaning that there was interruption to all but emergency endoscopic service with many endoscopists being recruited to other areas within the hospital. The interim solution of faecal immunochemical testing (FIT) to risk stratify patients with LS due to surveillance endoscopy was introduced. A cut-off of ≥10 µg/g faeces was prioritised for urgent colonoscopy,52 and subsequently patients in the UK have been recruited to an ongoing study to formally assess the role of FIT in this population.53
With the development of multiple testing platforms to identify early cancers, this population of patients with LS may benefit from other biomarkers such as cell-free DNA currently in early phases within the NHS.54 Identification of early cancers using non-invasive tools such as these can lead to downstaged diagnoses and improved clinical outcomes for patients.
Conclusion
Families with LS require a tailored approach to the lifelong surveillance of their condition. Robust research into beneficial, personalised surveillance is key to building patients’ confidence in extracolonic surveillance investigations over their lifetime; however, there is a clear benefit for 2-yearly, high-quality colonoscopy. Genetic counselling is essential to make sure families are well informed about their risks; however, for patients with newly diagnosed cancer, this may now be offered by gastroenterologists and other non-traditional genetics clinicians. As we diagnose more patients with LS, the impact of effective registration is vital. Overall, in recognition and identification of patients and families at risk of LS, diagnosis and intervention will help to work towards reduction in cancer burden in these patients.
Ethics statements
Patient consent for publication
Ethics approval
As a review article, it did not require ethics approval.
References
Footnotes
Twitter @kevinjmonahan
Contributors We have written this article in total, and this is original work.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.
Linked Articles
- UpFront