Abstract
Background/aim
The appearance of anti-adalimumab antibodies (AAAs) is associated with low serum adalimumab (ADA) trough levels and a decrease of clinical response. The goal of this study was to assess the accuracy and clinical utility of new immunoassays for serum ADA and AAA levels.
Patients and methods
Serum ADA trough levels and AAA levels were measured using new immunoassays in 40 patients with Crohn’s disease (CD) receiving ADA maintenance therapy.
Results
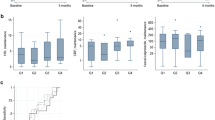
Serum ADA trough levels were 12.3 ± 9.6 μg/ml (n = 40) in CD patients, and 14 of 40 patients (35 %) were positive for AAAs. A negative correlation was observed between serum AAA levels and ADA trough levels (y = −6.02x + 18.7, r = −0.54, P < 0.001, n = 40). The ROC (receiver-operator curve) analyses indicated that an ADA trough of 5.9 μg/ml was optimal to maintain negative CRP (C-reactive protein) levels (≤0.3 mg/dl). The ADA trough levels were significantly lower in patients positive for AAAs (5.5 ± 5.4 μg/ml, n = 14) than in patients negative for AAAs (16.0 ± 9.5 μg/ml, n = 26). The CRP and ESR levels were significantly higher in AAA-positive patients than in AAA-negative patients. Serum albumin levels were significantly lower in AAA-positive patients. The positive rate for AAAs in patients who lost a response to infliximab (50 %) was significantly higher than that of anti-TNF-α drug naïve patients (12.5 %).
Conclusions
These new assays for serum AAA trough and AAA levels are useful for routine clinical use and may help guide selection of optimal management strategies for IBD patients with a loss of response to ADA.






Similar content being viewed by others
References
Danese S, Colombel JF, Reinisch W, et al. Review article: infliximab for Crohn’s disease treatment—shifting therapeutic strategies after 10 years of clinical experience. Aliment Pharmacol Ther. 2011;33:857–69.
Mayer L. Evolving paradigms in the pathogenesis of IBD. J Gastroenterol. 2010;45:9–16.
Hanauer SB, Kornbluth AA, Messick J, et al. Clinical scenarios in IBD: optimizing the use of conventional and biologic agents. Inflamm Bowel Dis. 2010;16(Suppl 1):S1–11.
Andoh A, Kuzuoka H, Tsujikawa T, et al. Multicenter analysis of fecal microbiota profiles in Japanese patients with Crohn’s disease. J Gastroenterol. 2012;47:1298–307.
Allez M, Vermeire S, Mozziconacci N, et al. The efficacy and safety of a third anti-TNF monoclonal antibody in Crohn’s disease after failure of two other anti-TNF antibodies. Aliment Pharmacol Ther. 2010;31:92–101.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–9.
Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
Ueno F, Matsui T, Matsumoto T, et al. Evidence-based clinical practice guidelines for Crohn’s disease, integrated with formal consensus of experts in Japan. J Gastroenterol. 2013;48:31–72.
D’Haens GR, Panaccione R, Higgins PD, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn’s and Colitis Organization: when to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011;106:199–212. quiz 213.
Cassinotti A, Travis S. Incidence and clinical significance of immunogenicity to infliximab in Crohn’s disease: a critical systematic review. Inflamm Bowel Dis. 2009;15:1264–75.
Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:542–53.
Vermeire S, Noman M, Van Assche G, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut. 2007;56:1226–31.
Imaeda H, Andoh A, Fujiyama Y. Development of a new immunoassay for the accurate determination of anti-infliximab antibodies in inflammatory bowel disease. J Gastroenterol. 2012;47:136–43.
Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2012;108(1):40–7.
Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–85.
Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut. 2009;58:492–500.
Van Assche G, Magdelaine-Beuzelin C, D’Haens G, et al. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology. 2008;134:1861–8.
Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760–7.
Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106:685–98.
Karmiris K, Paintaud G, Noman M, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn’s disease. Gastroenterology. 2009;137:1628–40.
Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–38.
West RL, Zelinkova Z, Wolbink GJ, et al. Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn’s disease. Aliment Pharmacol Ther. 2008;28:1122–6.
Chaparro M, Guerra I, Munoz-Linares P, et al. Systematic review: antibodies and anti-TNF-alpha levels in inflammatory bowel disease. Aliment Pharmacol Ther. 2012. doi:10.1111/j.1365-2036.2012.05057.x.
Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis. 2007;66:921–6.
Bartelds GM, Krieckaert CL, Nurmohamed MT, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305:1460–8.
Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol. 2011;106:674–84.
van Schouwenburg PA, Krieckaert CL, Rispens T, et al. Long-term measurement of anti-adalimumab using pH-shift-anti-idiotype antigen binding test shows predictive value and transient antibody formation. Ann Rheum Dis. 2013 [Epub ahead of print].
Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–44.
Sprakes MB, Hamlin PJ, Warren L, et al. Adalimumab as second line anti-tumour necrosis factor alpha therapy for Crohn’s disease: a single centre experience. J Crohns Colitis. 2011;5:324–31.
Orlando A, Renna S, Mocciaro F, et al. Adalimumab in steroid-dependent Crohn’s disease patients: prognostic factors for clinical benefit. Inflamm Bowel Dis. 2012;18:826–31.
Kiss LS, Szamosi T, Molnar T, et al. Early clinical remission and normalisation of CRP are the strongest predictors of efficacy, mucosal healing and dose escalation during the first year of adalimumab therapy in Crohn’s disease. Aliment Pharmacol Ther. 2011;34:911–22.
Sandborn WJ, Colombel JF, Schreiber S, et al. Dosage adjustment during long-term adalimumab treatment for Crohn’s disease: clinical efficacy and pharmacoeconomics. Inflamm Bowel Dis. 2011;17:141–51.
Afif W, Loftus EV Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:1133–9.
Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. Anti-adalimumab antibodies in rheumatoid arthritis patients are associated with interleukin-10 gene polymorphisms. Arthritis Rheum. 2009;60:2541–2.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (21590809) and by the Health and Labour Sciences Research Grants for Research on Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan.
Conflict of interest
The authors have declared that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Imaeda, H., Takahashi, K., Fujimoto, T. et al. Clinical utility of newly developed immunoassays for serum concentrations of adalimumab and anti-adalimumab antibodies in patients with Crohn’s disease. J Gastroenterol 49, 100–109 (2014). https://doi.org/10.1007/s00535-013-0803-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-013-0803-4