-
PDF
- Split View
-
Views
-
Cite
Cite
Kirsty R Short, Rebecca Veeris, Lonneke M Leijten, Judith M van den Brand, Victor L Jong, Koert Stittelaar, Ab D M E Osterhaus, Arno Andeweg, Debby van Riel, Proinflammatory Cytokine Responses in Extra-Respiratory Tissues During Severe Influenza, The Journal of Infectious Diseases, Volume 216, Issue 7, 1 October 2017, Pages 829–833, https://doi.org/10.1093/infdis/jix281
Close - Share Icon Share
Abstract
Severe influenza is often associated with disease manifestations outside the respiratory tract. While proinflammatory cytokines can be detected in the lungs and blood of infected patients, the role of extra-respiratory organs in the production of proinflammatory cytokines is unknown. Here, we show that both 2009 pandemic H1N1 influenza A (H1N1) virus and highly pathogenic avian influenza A (H5N1) virus induce expression of tumor necrosis factor α, interleukin-6, and interleukin-8 in the respiratory tract and central nervous system. In addition, H5N1 virus induced cytokines in the heart, pancreas, spleen, liver, and jejunum. Together, these data suggest that extra-respiratory tissues contribute to systemic cytokine responses, which may increase the severity of influenza.
Severe influenza virus infections are typically associated with a dysregulated proinflammatory cytokine response in the lungs and blood of infected individuals, a condition often referred to as a “cytokine storm” [1]. Compared to seasonal influenza A (H1N1) viruses, the highly pathogenic avian influenza A (HPAI) H5N1 virus is a more potent inducer of proinflammatory pulmonary cytokines in vitro and in vivo [2, 3].
In addition to causing a pronounced inflammatory response in the lungs and blood, severe influenza virus infections are associated with a wide range of extra-respiratory complications, including central nervous system and cardiovascular disease [4–6]. These extra-respiratory tract complications can be severe, and lead to multiorgan distress syndrome with fatal consequences [4]. Despite the involvement of extra-respiratory organs during severe influenza virus infections, no study to date has comprehensively examined the production of proinflammatory cytokines by these organs during the acute phase of infection. However, in mice, proinflammatory cytokines can be found in the central nervous system after influenza virus infection [7], and hypoxia-inducible factor-1 (a known regulator of the inflammatory response) is upregulated in the extra-respiratory tissues of macaques infected with influenza virus [8]. These studies suggest that extra-respiratory tissues respond to an influenza virus infection in the respiratory tract and contribute to a systemic proinflammatory cytokine response.
Among laboratory animals, ferrets are considered to be the “gold standard” for studying human influenza, even though in-depth studies are limited by the lack of available ferret-specific reagents. In a previous study, we have shown that HPAI H5N1 infection in ferrets resulted in higher morbidity and mortality compared to infection with 2009 H1N1 pandemic virus (pH1N1) [9]. In the present study, we sought to investigate if these differences in morbidity and mortality were associated with differences in extra-respiratory cytokine expression. Therefore, we assessed the expression of tumor necrosis factor α (TNFα), interleukin-6 (IL-6), and interleukin-8 (IL-8), which are known to play important roles in influenza virus pathogenesis in different organs of ferrets infected with the pH1N1 or HPAI H5N1 virus.
MATERIALS AND METHODS
Ferrets
Tissues from ferrets inoculated with pH1N1 (A/Netherlands/ 602/2009), HPAI H5N1 virus (A/Indonesia/5/2005), or Madin-Darby canine kidney cell lysate (mock) were obtained from a previous study [9] at 1 and 3 days postinfection (dpi). All samples collected during necropsy were immediately stored at –80°C. Samples were thawed once for homogenization for virus titrations. Tissues for histology were immediately fixed in formalin and processed 2–4 weeks later into paraffin. For the present study, 4 ferrets per time point were selected for ribonucleic acid (RNA) extraction (using frozen tissue homogenates) and in situ hybridization (using paraffin blocks) [9].
RNA Extraction and Complementary DNA Synthesis
RNA was extracted from the nasal turbinates, lung, olfactory bulb, cerebrum, spleen, liver, heart, kidney, pancreas, and jejunum homogenates using the High Pure RNA Isolation Kit (Roche LifeScience) and further purified using the Micro RNA Isolation Kit (Qiagen, Hilden, Germany) following manufacturer’s instructions. Complementary DNA was synthesized using SuperScript III Reverse Transcriptase (Invitrogen) and random primers.
Quantitative Polymerase Chain Reaction
Quantitative polymerase chain reaction (qPCR) for TNFα, IL-6, and IL-8 was performed using the Universal PCR Master Mix (Applied Biosystems). The respective primer/probe combinations are shown in Supplementary Table 1 [10]. The specificity of primer combinations was confirmed by Sanger sequencing of generated amplicons (data not shown). Reactions were performed on a 7500 Real-Time PCR System (Applied Biosystems). The delta cycle time (ΔCt; Ct gene of interest: Ct glyceraldehyde 3-phosphate dehydrogenase) were used to calculate the fold induction of individual cytokines. Fold inductions over mock-inoculated ferrets (log-fold changes) were calculated as the difference between the mean log expression values.
In Situ Hybridization
RNAScope RNA probes were custom designed by Advanced Cell Diagnostics (Hayward, CA) for ferret IL-6, IL-8, and TNFα. In tissues in which we observed a significant cytokine induction by qPCR, the cell type in which cytokine RNA was expressed was determined in tissue sections that were cut a maximum of 48 hours before the in situ hybridization procedure. Serial tissue sections were stained for histopathological analysis by hematoxylin and eosin. Cell types were identified by a European College of Veterinary Pathologist board-certified veterinary pathologist on morphological characteristics. The specificity of the probes was verified using a positive control probe ubiquitin C (UBC) and a negative control probe dihydrodipicolinate reductase (DapB-C4).
Statistical Analysis
Log-fold changes were calculated and differential expression analysis was performed using empirical Bayes linear models implemented in the R/Bioconductor software package limma. We controlled for multiple testing at a 5% false discovery rate using a Benjamini–Hochberg procedure.
RESULTS
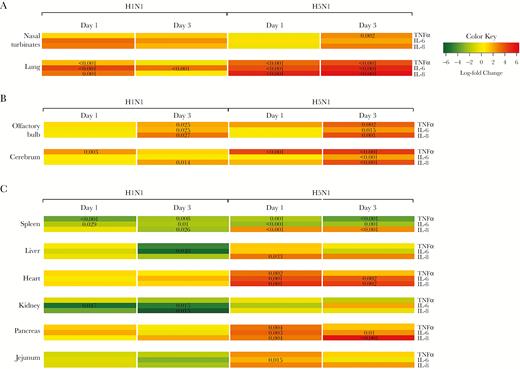
Increased Expression of TNFα, IL-6, and IL-8 in the Respiratory Tract of pH1N1 and H5N1 Virus-Inoculated Ferrets
In pH1N1 virus–inoculated ferrets, there was no significant upregulation of TNFα, IL-6, and IL-8 in the nasal turbinates relative to mock-infected ferrets (Figure 1A). In the lungs, TNFα, IL-6, and IL-8 were significantly upregulated at 1 dpi, and IL-6 at 3 dpi (Figure 1A). In situ hybridization showed that TNFα in the lungs was predominantly produced by macrophage-like cells and alveolar and bronchial epithelial cells; IL-6 was predominantly produced by epithelial cells of the bronchus, bronchioles, and alveoli; and IL-8 was predominantly expressed in neutrophils.
Proinflammatory cytokine expression in the respiratory organs (A), CNS (B), and extra-respiratory (C) organs of pH1N1 or HPAI H5N1 virus-infected ferrets at 1 and 3 days postinfection. Heat maps show gene expression relative to mock-infected ferrets. A P value for a specific gene and time point indicates the statistical significance. Abbreviations: CNS, central nervous system; HPAI, highly pathogenic avian influenza A; IL, interleukin; pH1N1, 2009 H1N1 pandemic virus; TNFα, tumor necrosis factor α.
In HPAI H5N1 virus–inoculated ferrets, only TNFα was significantly upregulated at 3 dpi in the nasal turbinates (Figure 1A). In the lungs, TNFα, IL-6, and IL-8 were significantly upregulated at 1 and 3 dpi (Figure 1A). TNFα was predominantly expressed in macrophage-like cells and few alveolar epithelial cells at 1 dpi, and in macrophage-like cells and bronchial epithelial cells at 3 dpi. IL-6 was predominantly expressed in epithelial cells of the bronchus, bronchioles, and alveoli, and IL-8 was predominantly expressed in neutrophils (Supplementary Figure 1). In general, cytokine expression in the lungs colocalized more with histological lesions (as described by [9]) than with expression of influenza virus antigen.
Increased Expression of TNFα, IL-6, and IL-8 in the Central Nervous System of pH1N1 and H5N1 Virus-Inoculated Ferrets
In pH1N1-inoculated ferrets, IL-6 was significantly upregulated in the cerebrum at 1 dpi (Figure 1B). At 3 dpi, IL-8 was significantly upregulated in the cerebrum, and TNFα, IL-6, and IL-8 were significantly upregulated in the olfactory bulb (Figure 1B). Only a few cells of undetermined origin were positive for the different cytokines by in situ hybridization.
In HPAI H5N1 virus–inoculated ferrets, TNFα was significantly upregulated at 1 dpi in the cerebrum, and TNFα, IL-6, and IL-8 were upregulated in the cerebrum and olfactory bulb at 3 dpi (Figure 1B). In situ hybridization showed that TNFα was expressed in a few small neurons or microglial cells in the brain stem at 1 dpi and 3 dpi; IL-6 was expressed in a few small neurons or microglial cells and meningeal cells of undetermined cell type at 3 dpi; and IL-8 was expressed in a few endothelial cells in the brain stem, and in a moderate number of cells in the olfactory bulb, cerebellum, and meninges at 3 dpi (Supplementary Figure 2). Histological lesions were not observed in the central nervous system (CNS) [9].
Expression of TNFα, IL-6, and IL-8 in Tissues Outside the Respiratory Tract and CNS of H1N1 and H5N1 Virus-Inoculated Ferrets
In pH1N1 virus–inoculated ferrets, there was no significant upregulation of the selected cytokines in tissues outside the respiratory tract and CNS (Figure 1C). However, at least 1 cytokine was downregulated in tissues of the spleen, liver, and kidney at 1 and/or 3 dpi (Figure 1C).
Ferrets inoculated with HPAI H5N1 virus showed a marked upregulation of 1 or more of the selected cytokines in the spleen, liver, heart, pancreas, and jejunum (Figure 1C). In the spleen, IL-8 was expressed in macrophage-like cells at 1 dpi, and in megakaryocytes and a few neutrophils at 3 dpi (Supplementary Figure 3). In the liver, IL-8 was expressed in Kupffer cells or fibroblasts and small cells, most likely endothelial cells at 1 dpi (Supplementary Figure 3). In the heart, TNFα was expressed in a few cardiomyocytes at 1 dpi; IL-6 was focally expressed in cardiomyocytes and macrophage-like cells at 1 dpi, and in endocardial cells, endothelial cells, fibroblasts, and some cardiomyocytes at 3 dpi; IL-8 was expressed in neutrophils and endocardial cells at 1 and 3 dpi (Supplementary Figure 4). In the pancreas, a few bile duct epithelial cells expressed TNFα at 1 dpi; IL-6 was expressed in scattered endothelial cells and fibroblasts at 1 dpi, and in endothelial cells, fibroblasts, and a few acinar cells at 3 dpi; IL-8 was expressed in a few endothelial cells, fibroblasts, bile duct cells, and acinar cells at 1 dpi, and in a few endothelial cells and acinar cells 3 dpi (Supplementary Figure 5). In general, cytokine expression did not colocalize with histological lesions, except in the heart, where IL-6 and IL-8 were associated with mild histological lesions, such as the degeneration and loss of striation of cardiomyocytes.
Extra-Respiratory Cytokine Production Correlates with the Presence of Infectious Virus in pH1N1-Infected Ferrets
In the original study, we showed that active virus replication, as measured by immunohistochemistry, was restricted to the respiratory tract [9]. In contrast, infectious virus could be isolated from multiple extra-respiratory tissues (Supplementary Table 2). Therefore, the association between extra-respiratory cytokine expression and the presence of infectious virus was determined. Unfortunately, this analysis was not possible on H5N1 virus–infected ferrets due to the presence of infectious virus in all extra-respiratory organs. However, in pH1N1 virus–infected ferrets, the presence or absence of infectious virus was significantly associated with the expression of the selected cytokines (point Biserial correlation; P = .02 [Day 1]; P = .043 [Day 3]).
DISCUSSION
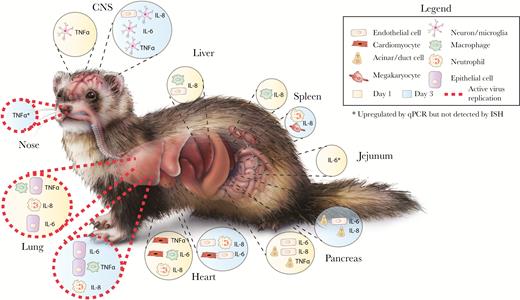
Here, we provide the first evidence that TNFα, IL-6, and IL-8 are upregulated in extra-respiratory tissues of ferrets early after infection with pH1N1 or HPAI H5N1 (Figure 2). Specifically, while inoculation with pH1N1 or HPAI H5N1 induced cytokine expression in the CNS, HPAI H5N1 inoculation induced a significant upregulation of the selected cytokines in the spleen, liver, heart, pancreas, and jejunum. In contrast, pH1N1 virus infection triggered a significant downregulation of proinflammatory cytokines in the spleen, liver, and kidney.
Schematic representation of cytokine induction in different organs and cells in HPAI H5N1 virus-infected ferrets at 1 and 3 days postinfection. Active virus replication refers to the detection of viral antigen by immunohistochemistry. Original content provider for ferret image: CDC. Original image available at: https://phil.cdc.gov/phil/home.asp, image number 22370.Abbreviations: CNS, central nervous system; H5N1, avian influenza A; HPAI, highly pathogenic avian influenza A; IL, interleukin; ISH, in situ hybridization; qPCR, quantitative polymerase chain reaction; TNFα, tumor necrosis factor α.
At present, the precise trigger of extra-respiratory cytokine production remains unclear. These data may reflect a systemic response to cytokines produced within the respiratory tract, which enter the circulation early after infection. It is also possible that extra-respiratory cytokine production is the result of local virus replication, even though we were not able to detect virus antigens in these extra-respiratory tissues by immunohistochemistry. However, it is interesting to note that extra-respiratory cytokine expression in pH1N1 virus–infected ferrets was associated with the presence of infectious virus. It is therefore tempting to speculate that during pH1N1 influenza virus infection, the virus spreads to extra-respiratory organs but fails to replicate efficiently in extra-respiratory organs. However, the mere presence of virus might be sufficient to induce a proinflammatory response.
The role of these extra-respiratory cytokines for disease severity remain to be determined, but it is striking to note that H5N1-infected ferrets, which suffered from severe disease, had more pronounced extra-respiratory cytokine production than pH1N1-infected ferrets [9]. Both H1N1 and H5N1 virus infection triggered cytokine responses in the CNS, and while extra-respiratory cytokine responses were often not associated with histological lesions, both TNFα and IL-6 are able to induce neuronal apoptosis [11].
Although influenza viruses can enter the CNS and cause local damage [12], these data suggest that CNS disease could also be the result of immune-mediated damage [6, 13]. For example, TNF is known to increase the permeability of the blood-brain barrier and induce cell death [14], and IL-6 production in the CNS has been associated with seizures [15].
Taken together, these data reveal that extra-respiratory tissues, in particular the CNS, actively contribute to proinflammatory cytokine responses during severe pH1N1 and fatal HPAI H5N1 virus infections. These data indicate an association between disease severity and extra-respiratory proinflammatory cytokine expression, raising the intriguing possibility that systemic proinflammatory cytokine response may increase the severity of influenza.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Peter van Run for excellent technical assistance and Thijs Kuiken for critical reading of the manuscript.
Financial support. This work was supported by a National Health and Medical Research Council (NHMRC) CJ Martin postdoctoral fellowship to K. R. S. (1054081) and by a fellowship from the Netherlands Organization for Scientific Research (contract 91614115) and a fellowship from the Erasmus University Medical Center Foundation to D. v. R. This work was further supported by the European Union Seventh Framework Programme for Research and Technological Development project ANTIcipating the Global Onset of Novel Epidemics (ANTIGONE; contract 278976).
Potential conflicts of interest. A.O. is part-time CSO at Viroclinics BV, and is an ad hoc consultant for public and private entities. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References