Article Text
Abstract
Background: Some patients with untreated coeliac disease are negative for serum endomysial autoantibodies (EmA) targeted against transglutaminase 2 (TG2).
Aims: To evaluate the clinical and histological features of EmA-negative coeliac disease, and to examine whether EmA-equivalent autoantibodies against TG2 can be seen in the small-bowel mucosa when absent in serum.
Patients: Serum EmA was studied in 177 biopsy-proved specimens from adult patients with coeliac disease. 20 patients with intestinal diseases served as non-coeliac controls; three had autoimmune enteropathy with villous atrophy.
Methods: Clinical manifestations, small-bowel mucosal morphology, intraepithelial inflammation and TG2-specific extracellular immunoglobulin A (IgA) deposits were investigated in both serum EmA-negative and EmA-positive patients.
Results: 22 patients with IgA-competent coeliac disease were negative for serum EmA. Three of these had small-bowel lymphoma. Patients with EmA-negative coeliac disease were older, had abdominal symptoms more often, and the density of γδ+ intraepithelial lymphocytes in their intestinal mucosa was lower than in EmA-positive patients; otherwise the histology was similar. All serum EmA-negative patients with coeliac disease, but none of the disease controls, had gluten-dependent mucosal IgA deposits alongside TG2 in the small-bowel mucosal specimens. In vivo deposited IgA was shown to be TG2-specific by its ability to bind recombinant TG2.
Conclusions: Negative serum EmA might be associated with advanced coeliac disease. TG2-targeted autoantibodies were deposited in the small-bowel mucosa even when absent in serum. This finding can be used in the diagnosis of seronegative coeliac disease when the histology is equivocal. It may also be helpful in the differential diagnosis between autoimmune enteropathy and coeliac disease.
- EATL, enteropathy-associated T cell lymphoma
- EmA, endomysial antibodies
- GFD, gluten-free diet
- GST, glutathione S-transferase-tagged
- HLA, human leucocyte antigen
- IEL, intraepithelial lymphocyte
- IgA, immunoglobulin A
- KSCN, potassium thiocyanate
- PBS, phosphate-buffered saline
- TG2, transglutaminase 2
- U, unit value
Statistics from Altmetric.com
- EATL, enteropathy-associated T cell lymphoma
- EmA, endomysial antibodies
- GFD, gluten-free diet
- GST, glutathione S-transferase-tagged
- HLA, human leucocyte antigen
- IEL, intraepithelial lymphocyte
- IgA, immunoglobulin A
- KSCN, potassium thiocyanate
- PBS, phosphate-buffered saline
- TG2, transglutaminase 2
- U, unit value
Small-bowel mucosal villous atrophy and crypt hyperplasia remain the golden standard in the diagnosis of coeliac disease.1 However, coeliac disease has no pathognomic histological features,2,3 and diagnosis can be difficult especially in the presence of borderline histology. Serology clearly has a supportive role,1 as a specific feature in coeliac disease is the presence of serum immunoglobulin A (IgA)-class endomysial antibodies (EmA) targeted against transglutaminase 2 (TG2). There is some controversy concerning the interpretation of negative EmA in the serum of patients suspected of having coeliac disease.4,5 In obscure cases, a histological or clinical response to a gluten-free diet (GFD) or a laborious and time-consuming gluten challenge is required to ascertain the diagnosis.5
Although a positive serum EmA has a close to 100% specific association with coeliac disease,6 approximately 10–20% of patients with untreated coeliac disease remain negative for serum EmA.7,8 On the other hand, when patients with negative serum EmA and borderline histological lesions are treated with a GFD, there is always a possibility for a false diagnosis of coeliac disease.3,5 Data suggesting whether EmA negativity is related to a specific clinical or histological course of coeliac disease are conflicting. Most studies suggest that EmA negativity is commonly associated with mild histological lesions,9–11 which would contradict the notion that EmA is a marker for early-stage coeliac disease without obvious villous atrophy.12
EmA-binding patterns in serum samples from patients with coeliac disease have proved to be exclusively TG2-targeted,13,14 and the correlation between EmA and TG2 antibodies is therefore good.15,16 Evidence shows that coeliac autoantibodies are produced in the small-bowel mucosa. In phage antibody libraries from the peripheral and intestinal lymphocytes of patients with coeliac disease, the humoral response against TG2 was shown to occur at the local level in the intestinal mucosa but not peripherally.17 This has also been shown by detecting EmA in duodenal biopsy organ culture supernatants from patients with untreated coeliac disease, and also from patients with treated coeliac disease after in vitro gliadin challenge.18 The concept of local production of coeliac autoantibodies was reinforced in our previous study showing the presence of TG2-targeted extracellular IgA deposits detected by direct immunofluorescence from the small-bowel mucosa of patients with untreated coeliac disease.19,20 It is intriguing to hypothesise that TG2-targeted autoantibodies would be present in the small-bowel mucosa of patients with untreated coeliac disease even when serum autoantibodies (EmA) are not detectable.
Our study aimed to compare the clinical and histological features of IgA-competent serum EmA-negative patients with coeliac disease with those in EmA-positive patients. Further, we investigated whether TG2-specific IgA deposits can be found in the small-bowel mucosa even in seronegative patients with coeliac disease. This would have a diagnostic value in EmA-negative people suspected of coeliac disease yielding ambiguous histology, and would in most cases make the laborious gluten challenge unnecessary.
MATERIALS AND METHODS
Patients and controls
The participants were enrolled from among 833 consecutive adult patients who underwent upper gastrointestinal endoscopy at Tampere University Hospital, Tampere, Finland, between 1995 and 2000 because of suspicion of coeliac disease. Endoscopy and small-bowel biopsy were performed when coeliac disease was suspected regardless of the antibody result. Villous atrophy and crypt hyperplasia compatible with coeliac disease1 were found in 177 of 833 (21%) patients. Patients with selective IgA deficiency were excluded from further evaluations. Signs and symptoms leading to suspicion of coeliac disease, family history of coeliac disease and the number of patients deceased after the diagnosis of coeliac disease were recorded. For the examination of small-bowel mucosal TG2-targeted IgA deposits and for the comparison of histological response to GFD, an age-matched and sex-matched EmA-positive patient with coeliac disease was selected for each IgA-competent EmA-negative patient with coeliac disease.
In all, 20 patients with intestinal disorders, but not with coeliac disease, served as controls, three of whom had autoimmune enteropathy-evinced villous atrophy (negative for human leucocyte antigen (HLA) DQ2 and DQ8), and the remaining 11 with dyspepsia, 3 with collagen colitis, 2 with ulcerative colitis and 1 with Crohn’s disease had normal villous architecture. All 20 controls were negative for serum IgA-class EmA.
Serology
Serum IgA-class EmA samples were measured in the same laboratory. An indirect immunofluorescence method was used with human umbilical cord as substrate; a dilution of 1:⩾5 was considered to be positive. Positive and negative controls were included in every test batch.6 Assessment of serum IgA-class TG2 antibodies was carried out by ELISA using guinea pig liver TG215 (Inova Diagnostics, San Diego, California, USA; a unit value (U) ⩾20 U being positive) and human recombinant TG216 (Celikey, Pharmacia Diagnostics, GmbH, Freiburg, Germany; ⩾5 U positive) as antigens.
Small-bowel mucosal morphology and inflammation
On endoscopy, seven forceps biopsy specimens were taken from the distal part of the duodenum. Five were processed, stained with haematoxylin and eosin, and studied under light microscopy. The specimens were interpreted according to the criteria of Marsh.11 Marsh III lesion was further classified into three subgroups: Marsh IIIa indicated severe partial, Marsh IIIb subtotal and Marsh IIIc total villous atrophy. In addition, to study the mucosal histology more objectively, the villous height:crypt depth ratio was determined from well-oriented biopsy samples from multiple sites.21 A ratio <2 was considered to be compatible with coeliac disease.
Two small-bowel biopsy specimens were freshly embedded in optimal cutting temperature compound (Tissue-Tec, Miles Elkhart, Indiana, USA), snap-frozen in liquid nitrogen and stored at −70°C. Immunohistochemical stainings for CD3+ and γδ+ intraepithelial lymphocyte (IEL) densities were determined as described previously.20,22 The reference values were set at 37 cells/mm for CD3+ and at 4.3 cells/mm for γδ+ IELs.23 In our laboratory, the correlation coefficients for intraobserver variation for CD3+ and γδ+ IELs were 0.95 and 0.98, and those for interobserver variation 0.92 and 0.98, respectively.
Small-bowel mucosal TG2-targeted IgA deposits
In earlier studies, we have shown that EmA-positive patients with coeliac disease have in vivo in situ IgA deposits on TG2 in their small-bowel mucosa, and when this IgA was eluted from the tissues, it targeted purified TG2 both in ELISA and in western blot.19 The method used here was based on our previous experiments to detect TG2-specific antibodies in situ in tissue sections by their colocalisation with TG2 when double labelled by immunofluorescence.
Frozen duodenum specimens were available in 18 of 22 EmA-negative and 17 of 22 EmA-positive patients with coeliac disease, and in all 20 controls. From each of these patients, altogether six unfixed, 5-µm-thick sections from frozen small-bowel specimens were processed, three for investigating IgA deposits and three for double-colour labelling for both IgA and TG2. IgA was detected by direct immunofluorescence using fluorescein isothiocyanate-labelled rabbit antibody against human IgA (Dako AS, Glostrup, Denmark) at a dilution of 1:40 in phosphate-buffered saline (PBS), pH 7.4. In coeliac disease, a clear subepithelial IgA deposition can be found below the basement membrane along the villous and crypt epithelium and around mucosal vessels; this is in contrast with normal small-bowel samples, where IgA is detected only inside the plasma and epithelial cells.19,20 These coeliac disease-type IgA deposits were graded from 0 to 3 on the basis of the intensity along basement membranes in the villous–crypt area. The evaluation was carried out blinded to the disease history or laboratory findings. For the double labelling, sections were stained for human IgA (green, as above) and for TG2 (red) using monoclonal mouse antibodies against TG2 (CUB7402, NeoMarkers, Fremont, California, USA) followed by rhodamine-conjugated anti-mouse Ig antibodies (Dako), both diluted 1:200 in PBS. More than 500 small-bowel specimens have been investigated for IgA deposits in our laboratory so far, and intraobserver and interobserver variations have both been 98% in the detection of the presence or absence of TG2-targeted IgA deposits between five investigators.
Investigation of target specificity of small-bowel mucosal IgA deposits
Unfixed frozen duodenum sections from seven serum EmA-negative and six EmA-positive patients with coeliac disease were washed in PBS, pH 7.4, and incubated for 30 min with 0.1 M sodium citrate buffer (pH 5) or with 0.5–1 M potassium thiocyanate (KSCN), which dissolves non-specific protein complexes as a chaotropic agent.24 After further washing in PBS, the sections were stained for human IgA and TG2 as described in the previous section.
In further experiments, extracellular TG2 was removed from the sections using 0.25% chloroacetic acid (Fluka Chemie AG, Buchs, Switzerland) in 0.2 M NaCl, pH 2.7, after treatment with KSCN. Chloroacetic acid is needed to disrupt the tight binding of TG2 to fibronectin25 and to remove TG2 from the tissues.19 The sections were thereafter similarly stained for remaining IgA and TG2.
To prove that extracellular IgA deposits in the small bowel of EmA-negative patients with coeliac disease was targeted against TG2, we investigated whether it would bind labelled TG2 added to the tissue. Glutathione S-transferase-tagged full-length human recombinant TG2 (GST-TG2) was expressed in Escherichia coli as described previously.26 Unfixed frozen small-bowel sections from patients with coeliac disease and controls were washed in PBS and incubated for 15 min at room temperature with GST-TG2 at a concentration of 0.01 mg/ml. After extensive washing, GST-TG2 bound to the tissue was labelled red by goat antibodies against GST (Pharmacia Biotech, Uppsala, Sweden) followed by Alexa Fluor 594-conjugated chicken antibodies against goat immunoglobulins (Molecular Probes, Leiden, The Netherlands). Human IgA in the tissue was labelled green as described previously. The anti-GST antibody used did not cross react with natural TG2 in the tissues. To block the binding of GST-TG2 to tissue fibronectin, GST-TG2 was also added to the sections with the 45-kDa gelatine-binding fragment of human fibronectin (Sigma F-0162, Sigma-Aldrich, St Louis, Missouri, USA; 0.2 mg/ml) and monoclonal antibodies G92 (0.4 mg/ml).27 These antibodies recognise the blocked N-terminal segment of TG2 with high specificity.
HLA typing
HLA DQB1* allele groups were investigated using the Olerup SSP DQ low-resolution kit (Olerup SSP AB, Saltsjöbaden, Sweden). This method determines HLA DQ2, DQ4, DQ5, DQ6, DQ7, DQ8 and DQ9 allele groups.
Statistical analysis
Quantitative data were expressed as medians and ranges. Statistical differences between study groups were evaluated using Pearson’s χ2 test, Fisher’s exact test or Mann–Whitney U test, as appropriate. Values of p<0.05 were considered to be significant.
Ethical considerations
The study protocol was approved by the ethics committee of Tampere University Hospital and informed consent was obtained from all study participants.
RESULTS
Of the 177 patients with coeliac disease, 26 (15%) had negative serum EmA, and 4 of these were IgA-deficient. Thus, 22 EmA-negative patients with coeliac disease constituted the study group. HLA DQ2 or DQ8 was detected in each of 12 patients with available sample (HLA DQ2 in 11 and DQ8 in 1). Among EmA-negative patients with coeliac disease, 13 (59%) were men and the median age was higher than in EmA-positive patients (table 1).
Demographic data and signs and symptoms leading to suspicion of coeliac disease in 173 immunoglobulin A-competent patients with coeliac disease
Abdominal symptoms were significantly more common in the EmA-negative group. Three EmA-negative patients with coeliac disease were found to have enteropathy-associated T cell lymphoma (EATL), which was detected at the same time as the diagnosis of coeliac disease was established. Two of these patients had HLA DQ2 and in one there were no data available. All three patients had proximal small-bowel villous atrophy and crypt hyperplasia compatible with coeliac disease while on a gluten-containing diet. Furthermore, two of these patients had small-bowel biopsy taken earlier, 2 and 6 years before the diagnosis of coeliac disease and EATL. Even at that time, both showed partial villous atrophy and crypt hyperplasia, but the diagnosis of coeliac disease was overlooked. In all, 6 (27%) of the 22 EmA-negative patients and 6 (4%) of the 151 EmA-positive patients with coeliac disease died after the diagnosis of coeliac disease.
No differences were observed between EmA-negative and EmA-positive patients with coeliac disease in Marsh classification (table 2) or villous height:crypt depth ratios (fig 1).
Severity of small-bowel mucosal villous atrophy according to Marsh classification in 173 immunoglobulin A-competent patients with coeliac disease
Villous height:crypt depth ratios in immunoglobulin A (IgA)-competent endomysial antibody (EmA)-negative and EmA-positive patients with coeliac disease. Median values are shown by solid lines. In the EmA-negative group, filled squares denote transglutaminase 2 (TG2) antibody-negative patients, open squares TG2 antibody-positive patients and filled ellipses patients without available TG2 antibody result. Furthermore, EmA-negative patients with small-bowel lymphoma are indicated with an arrow.
The median density of CD3+ IELs (fig 2A) was similar (p = 0.292), whereas the density of γδ+ IELs was statistically significantly higher (p = 0.007) in EmA-positive than in EmA-negative patients (fig 2B). Of the three EmA-negative patients with EATL, two had normal densities of γδ+ IELs.
The density of CD3+ (A) and γδ+ (B) intraepithelial lymphocytes (IELs) in immunoglobulin A (IgA)-competent endomysial antibody (EmA)-negative and EmA-positive patients with coeliac disease. The reference values were 37 cells/mm of epithelium for CD3+ and 4.3 cells/mm for γδ+ IELs. Median values of IELs are shown by solid lines. In the EmA-negative group, filled squares denote transglutaminase 2 (TG2) antibody-negative patients, open squares TG2 antibody-positive patients and filled ellipses patients without available TG2 antibody result. EmA-negative patients with small-bowel lymphoma are indicated by arrows.
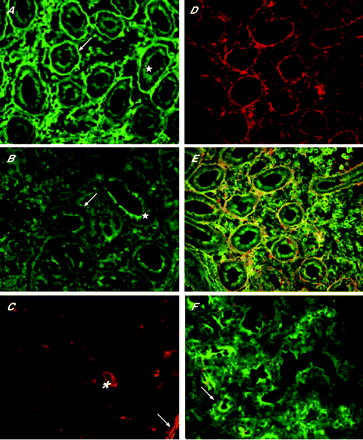
Small-bowel mucosal IgA deposits in colocalisation with extracellular TG2 were detected in all EmA-negative (n = 18) and EmA-positive (n = 17) examined patients with coeliac disease (figs 3 and 4). The intensity of intestinal IgA deposits did not correlate with the severity of the mucosal lesion—that is, villous height:crypt depth ratios. For example, three EmA-negative patients with coeliac disease with villous height:crypt depth ratios close to 1.5 had IgA deposits with 2.5+ to 3+ intensity. Figure 4 shows that the intensity of mucosal TG2-targeted IgA deposits decreased after adopting a GFD. In contrast, TG2-targeted IgA deposits were not detected in any of the controls with intestinal diseases, not even in patients having autoimmune enteropathy with severe villous atrophy (fig 4).
Subepithelial coeliac-type small-bowel mucosal immunoglobulin A (IgA) deposits (A, D, green, arrow) in IgA-competent patients with coeliac disease, with human leucocyte antigen DQ2 and negative serum endomysial (EmA) and transglutaminase 2 (TG2) antibodies. Yellow colour in composite pictures (B, E) indicates colocalisation of coeliac-type IgA deposits (green) and TG2 (red). Composite pictures of small-bowel biopsy specimens of a patient with autoimmune enteropathy having villous atrophy (C, F); IgA deposition or colocalisation of IgA and TG2 (yellow) was not detected. Bar = 50 µm.
Transglutaminase 2 (TG2)-specific immunoglobulin A (IgA) deposits in the small-bowel mucosa of IgA-competent, endomysial antibody (EmA)-negative patients with coeliac disease and age-matched and sex-matched EmA-positive patients with coeliac disease on a normal gluten-containing diet (GCD) and after a median of 1 year on a gluten-free diet (GFD). All available intestinal IgA deposit results are shown; some patients had results available only while on GCD or while on GFD. Patients with intestinal diseases other than coeliac disease and maintaining a GCD served as controls. Open ellipses denote EmA-negative patients with coeliac disease and small-bowel lymphoma.
To obtain direct evidence of the TG2 specificity of IgA deposits, further experiments were carried out. The small-bowel mucosal subepithelial and pericryptal IgA deposits along TG2 in both EmA-negative and EmA-positive patients with coeliac disease remained unchanged after treatment with citrate buffer and 0.5–1 M KSCN (fig 5A). In contrast, the amount of IgA deposits substantially decreased in eight samples and almost completely disappeared in five samples (fig 5B) when the sections were treated additionally with chloroacetic acid, which removes TG2 from its fibronectin binding sites. The amount of detectable TG2 also decreased in parallel (fig 5C), whereas IgA in the brush border of epithelial cells remained essentially unchanged (fig 5A, B; asterisks). Chloroacetic acid had similar effects in EmA-negative and EmA-positive samples.
Investigation of specificity of deposited immunoglobulin A (IgA) for transglutaminase 2 (TG2). (A) Pretreatment of coeliac duodenum section with 0.5 M potassium thiocyanate (KSCN) does not affect extracellularly deposited IgA (green). After incubation with chloroacetic acid, which removes intrinsic TG2 from its fibronectin-binding sites, most deposited IgA (B) and extracellular TG2 (red, C) disappear from the sections (arrows), but IgA in the epithelial cells remains unchanged (A, B; asterisks). Some TG2 is visible only in vessels (C; asterisk) and smooth-muscle cells (arrow, for comparison see panel F in fig 3). Exposure time for B and C was four times longer than for A. (D, E) Glutathione S-transferase-tagged full-length human recombinant TG2 (GST-TG2) shown in red by anti-GST antibodies binds to coeliac duodenum section (D) along extracellularly deposited IgA, shown in green (E). Merging of green and red labels to yellow indicates colocalisation. Direct binding of GST-TG2 to fibronectin was blocked with 45 kDa fibronectin fragment and with monoclonal antibodies specifically targeted against the N-terminal epitope of TG2. (A–E) Specimens are from the same endomysial antibody-negative patient with coeliac disease as in fig 3. (F) No binding of GST-TG2 to control duodenum without IgA deposition, double stained for IgA (green) and GST-TG (red). Arrow shows the crypt region. Natural TG2 in the tissue is not recognised by the anti-GST antibody. Bar = 50 µm.
When the small-bowel sections were incubated in vitro with human recombinant GST-TG2, binding of GST-TG2 was observed both in coeliac and in non-coeliac tissue sections along fibronectin (data not shown). This non-specific binding to fibronectin could be blocked by pre-incubating GST-TG2 with a soluble 45-kDa fragment of fibronectin as well as the G92 monoclonal anti-TG2 mouse antibodies. Under these conditions, GST-TG2 bound only to the coeliac tissue, colocalising with the IgA deposits (fig 5D,E), but did not bind to the duodenum sections from controls without extracellular IgA deposition (fig 5F). Small-bowel sections from the seven serum EmA-negative patients with coeliac disease gave similar results as the six EmA-positive coeliac samples. These experiments collectively show that coeliac IgA antibodies were specifically bound in situ to TG2 target antigen in the duodenum samples of both serum EmA-negative and EmA-positive patients with coeliac disease.
Serum TG2 antibody test results were available in 14 of 22 EmA-negative patients with coeliac disease; five were tested using guinea pig liver and nine using human recombinant as antigen. Four were positive and 10 negative for TG2 antibodies. Three of four EmA-negative TG2 antibody-positive patients had only low TG2 antibody levels using human recombinant as antigen (5.4, 6.8 and 12.8 U; normal values <5 U, median titre in untreated patients with coeliac disease 70.3, range 8.8–680).16 Only one had a high TG2 antibody level using guinea pig liver as antigen (159 U, normal value <20 U). In the EmA-negative group, patients having positive TG2 antibodies in the serum did not show more intense intestinal IgA deposits than in TG2 antibody-negative patients.
After a median of 13 months on a GFD, there were no differences in small-bowel histological recovery between EmA-negative and EmA-positive patients with coeliac disease. Histological improvement was observed in all patients who underwent small-bowel biopsy while on a GFD, except in the three affected by EATL. Three EmA-negative and four EmA-positive patients did not undergo small-bowel biopsy, but clinical recovery on a GFD was evident in all; one EmA-negative patient and one EmA-positive patient were lost to follow-up.
DISCUSSION
This study yielded two major findings: firstly, EmA-negative patients with coeliac disease were older and had more abdominal symptoms and complications than EmA-positive patients, which suggest that they had more advanced coeliac disease. Secondly, even when autoantibodies (EmA) against TG2 were not measurable in the serum, TG2-specific gluten-dependent autoantibodies were deposited and detectable in the small-bowel mucosa in all patients with coeliac disease.
The frequency of EmA-negative coeliac disease has been markedly different in previous studies.6,11,28,29 These divergences are obviously dependent on the populations tested and on the likelihood of the disease. Further, there exists a possibility of selection bias, as EmA-negative patients are less likely than EmA-positive patients to be examined rigorously for coeliac disease. This might be an explanation for the more evident clinical manifestations in EmA-negative patients with coeliac disease in this study. On the other hand, in our department small-bowel biopsy was taken every time the patient underwent endoscopy, regardless of the indication, and endoscopy was performed in all patients suspected of coeliac disease, and also in seronegative cases. The prevalence figure (15%) for EmA-negative coeliac disease in our cohort was comparable to that reported in many other studies,7,28,29 indicating that our series would be representative. Most EmA-negative patients were men (table 1). However, 117 (66%) of all 177 patients with coeliac disease in the present series were female, which is the typical sex distribution found in coeliac disease. Thus, the male predominance seems to be a true finding rather than a result of selection bias.
It has been proposed that coeliac autoantibodies might have a biological role in the immunopathology of the coeliac mucosal lesion,30 but the fact that these autoantibodies are not present in the serum of every patient with coeliac disease contradicts this concept.31,32 The current study does not exclude the possible importance of autoantibodies in the pathogenesis of coeliac disease, as we showed that autoantibodies (equivalent to EmA) targeted against TG2 were deposited in the small-bowel mucosa of even seronegative patients with coeliac disease, and also that these deposits were gluten dependent. Moreover, we could also show that the in vivo deposited IgA is functional towards TG2, as it was also able to bind externally added recombinant human TG2. Thus, it appears that autoantibodies seem to be sequestered in the bowel of seronegative patients and autoantibodies present in the serum seem to be caused by spill-over from the gut. IgA antibodies of EmA-negative patients could not be removed from the gut tissue by moderate amounts of KSCN. As KSCN is often used to test the avidity of antigen–antibody binding,24 our results also indicate that coeliac antibodies are bound to intestinal TG2 with considerably high avidity. During a longstanding immune reaction, antibodies with increasing avidity are produced, which makes it understandable why older patients with coeliac disease may have lower serum EmA levels than the younger ones. Thus longstanding coeliac disease might even result in seronegativity.
Some uncertainty often exists in the diagnosis of coeliac disease when serum EmA is negative, as villous atrophy can also be present in other disorders.3–5 Also, the poor quality of biopsy specimens makes erroneous diagnosis possible.28 In this study, none of the EmA-negative patients with coeliac disease were HLA DQ2 and DQ8 negative, and histological or clinical recovery on a GFD was shown. The presence of small-bowel mucosal TG2 autoantibodies eventually confirmed the diagnosis of coeliac disease in EmA-negative patients. The absence of intestinal TG2-targeted autoantibodies in controls, especially in patients with autoimmune enteropathy and villous atrophy, is certainly of value in the differential diagnosis between autoimmune enteropathy and coeliac disease.
The older age and more severe clinical symptoms of EmA-negative patients with coeliac disease compared with EmA-positive patients suggest that coeliac disease has remained unrecognised for a long time in EmA-negative people. The disappearance of gliadin antibodies from the serum of patients with coeliac disease who had discontinued their GFD for a long time has been shown previously .33 Further, the lack of humoral immune response typical of coeliac disease in patients with EATL has also been seen.34 In the present study, negative EmA in three patients with untreated coeliac disease with EATL also supports the conclusion that EmA negativity is connected with longlasting, severe disease. We emphasise that the normal density of γδ+ IELs in two patients with EATL does not exclude coeliac disease. The sensitivity of γδ+ IELs in the diagnosis of coeliac disease has been shown to be 93–94%.23,35 In patients with coeliac disease and EATL, the sensitivity may be even lower; rearrangement in the T cell receptor γ gene, with low densities of γδ+ IELs, has been documented in patients with refractory sprue or EATL.36,37 On the other hand, an increased density of γδ+ IELs is not restricted to HLA DQ2 or DQ838 and hence is not a finding specific for coeliac disease.23
Collection of the current data began in 1995, and TG2 was not identified as the main and probably the sole autoantigen for EmA until 1997.39 EmA and TG2 antibody tests correlate closely,15,16 and the sensitivity and specificity values of these tests have been equal. Regardless of that, some patients with coeliac disease positive for EmA remain negative for TG2 antibodies and vice versa. One explanation for this fact could be that EmA and TG2-ELISA test systems expose TG2 antigenic epitopes in different ways. In this study, retrospective measurement of TG2 antibodies was not possible in every patient, but in those tested TG2 antibodies were increased in only one third of the EmA-negative patients with coeliac disease. Thus the detection of serum TG2 antibodies did not solve the problem of EmA-negative coeliac disease, as also shown previously.40
CONCLUSIONS
Our study suggests that serum EmA negativity might be related to a longlasting, complicated coeliac disease. Further, the results indicate that EmA-negative patients with coeliac disease had gluten-dependent TG2-specific IgA deposits in the small-bowel mucosa, which were not detected in any of the controls. The presence of these intestinal autoantibodies strengthens the diagnosis of coeliac disease. We suggest that this method could be used in the diagnostic investigation of seronegative coeliac disease instead of the time-consuming and laborious follow-up or gluten challenge, and also in the differential diagnosis of autoimmune enteropathy.
Acknowledgments
This study was supported by the Research Fund of the Finnish Coeliac Society, the Medical Research Fund of Tampere University Hospital, the Finnish Medical Foundation, the Foundation for Paediatric Research in Finland, the National Graduate School of Clinical Investigation, the Finnish Foundation of Gastroenterological Research, Yrjo Jahnsson foundation and the Finnish Medical Society Duodecim.
REFERENCES
Footnotes
-
Published Online First 29 March 2006
-
Funding: This study was supported by the Research Fund of the Finnish Coeliac Society, the Medical Research Fund of Tampere University Hospital, the Finnish Medical Foundation, the Foundation for Paediatric Research in Finland, the National Graduate School of Clinical Investigation, the Finnish Foundation of Gastroenterological Research, Yrjo Jahnsson Foundation and the Finnish Medical Society Duodecim.
-
Competing interests: None.
Linked Articles
- Digest