Article Text
Abstract
Gastroparesis is defined by the presence of delayed gastric emptying (GE) in the absence of mechanical obstruction. Symptoms that have been attributed to gastroparesis include postprandial fullness, early satiation nausea and vomiting. Gastroprokinetic drugs are the preferred treatment option. A number of problems with the concept of gastroparesis have been identified recently. Major overlap exists with the symptom complex of the functional dyspepsia subtype of postprandial distress syndrome. The distinguishing feature of gastroparesis is delayed GE, but the correlation between delayed emptying and symptom pattern or severity in gastroparesis is modest and the stability of delayed emptying over time is poor. Other pathophysiological mechanisms such as hypersensitivity or impaired accommodation may also underlie symptoms in patients with gastroparesis. Moreover, symptomatic response to prokinetic therapy is variable and cannot be predicted based on the degree of enhancing GE. A number of approaches have been proposed to increase clinical usefulness of a diagnosis of gastroparesis, including a higher threshold of abnormal emptying and selection of patients with a specific symptom pattern more likely to be associated with delayed emptying.
- FUNCTIONAL DYSPEPSIA
- GASTRIC EMPTYING
- GASTROPARESIS
- PROKINETIC AGENT
Statistics from Altmetric.com
Gastroparesis: current defintions, concepts and implications
Introduction
The correlation between digestive symptoms suggestive of gastroduodenal disorders and delayed gastric emptying (GE) has long been debated, and this issue has attracted the interest of investigators and experts in the field with variable degree of interest in different times over the last 20 years,1 but it has remained unsolved to date. A revitalised interest has recently risen, after the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) gastroparesis clinical consortium gathered a number of investigators in US with access to scintigraphic techniques for measuring GE times.2–4
Definitions
The term gastroparesis is currently used to define a syndrome characterised by delayed GE, in the absence of mechanical obstruction, associated with one or more of the following symptoms: postprandial fullness, early satiety, nausea, vomiting and bloating.2 Small variations exist on the symptoms included in the definition. In some publications bloating is described as abdominal discomfort,3 while, despite the fact that postprandial fullness is one of the most frequent symptoms of patients both in USA5 and in Europe,6 in some publications on gastroparesis it is not considered as an individual symptom, but only in association with bloating,7 or with early satiety probably due to a misinterpretation of one of the subscales of the gastroparesis cardinal symptom index (GCSI),8 or even not included at all in the list of symptoms of gastroparesis.9 ,10 Thus, the definition of the syndrome varies, although slightly, in different publications, primarily according to slightly different symptom questionnaires adopted. Gastroparesis can occur in several clinical settings, in particular as a complication of diabetes mellitus.11 Other potential aetiologies include post-surgery, Parkinson's disease, collagen vascular disorders, intestinal pseudo-obstruction, viral infections, drugs etc.,7 ,12 but in the majority of cases no underlying causes can be found and gastroparesis is defined as idiopathic. Gastroparesis can be further classified according to the severity of symptoms (and not to the degree of delay in GE) into three severity subgroups: (a) mild gastroparesis (symptoms easily controlled and able to maintain weight and nutrition on a regular diet), (b) compensated gastroparesis (moderate symptoms with only partial control with use of daily medications, maintain nutrition with dietary adjustments), (c) gastric failure (refractory symptoms that are not controlled, inability to maintain oral nutrition, emergency department visits, frequent physician visits, or hospitalisations).13
GE testing
A causative role played by delayed GE in the determinism of the associated symptoms is implicit in the definition of gastroparesis,14 although other potential pathophysiological mechanisms underlying the same symptoms have been described, including abnormal fundic accommodation,15 diffuse GI neuro-myopathies such as in the case of chronic intestinal pseudo-obstruction,16 and even accelerated GE.17 Indeed, delay of GE is per se a very non-specific finding, since it can be detected in a variety of organic, systemic, metabolic and psychiatric diseases and may or may not be associated with digestive symptoms. Also different forms of acute and chronic stressors are associated with altered GI motility in general and delayed GE, in particular.18 ,19 Scintigraphy is currently considered the gold-standard for measuring GE. In order to overcome lack of reproducibility of different radiolabelled test meals and different recording techniques adopted in different centres, consensus standards have been internationally developed20 and later adopted by the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine.21 The recommended protocol involves a standardised, low-fat, egg-white technetium-99m sulfur colloid radio-labelled meal. In order to save expensive gamma-camera time, recording is recommended only at 0, 1, 2 and 4 h after meal ingestion and delayed GE is defined if there is ≥90% gastric retention at 1 h, ≥60% at 2 h and ≥10% at 4 h. Several factors can influence GE times, including stress, medications, smoking and hyperglycaemia and should be carefully standardised. Relevant limitations of the technique need to be taken into consideration: (a) due to the radiation exposure, the technique is not ideal for repeated measurements in the same subject, which dampen its applicability in pathophysiological studies and clinical trials; (b) the proposed test meal is small, since patients with severe forms of gastroparesis may be unable to eat larger meals, but the limited amount of caloric content (255 kcal; 72% carbohydrate, 24% protein, 2% fat and 2% fibre) is borderline to convert fasting into fed GI motor activities in healthy subjects22 and certainly may underestimate delayed GE in milder cases; (c) the lack of lipids is of particular concern as many patients with gastroparesis indicate worsening of symptoms with fatty meals; if fat contributes to pathogenesis or symptom manifestation, this meal will miss it; (d) normal values should be evaluated separately in large groups of healthy males and females, since gastric empting is delayed in healthy females, compared with age-matched healthy males.1
Other, less invasive techniques are available for measuring GE. Breath testing involves non-radiating 13C isotope bound to a stable substance, such as octanoic acid, acetate or Spirulina platensis (algae) and is suitable for repeated testing. 13C-labeled octanoic acid is more commonly used, and has been demonstrated to indirectly measure the gastric-emptying rate of solids with similar reliability and reproducibility of scintigraphy.23–25 Standardised meals are available for GE breath testing26 whose main disadvantages are twofold: (a) the proposed test meals are characterised by low-caloric contents, thus facing the same drawbacks of the standardised scintigraphic technique; (b) its results are unreliable in patients with malabsorption or liver failure.
Epidemiology
Only scanty data are available on the epidemiology of gastroparesis, since the scintigraphic technique that is considered the gold standard for measuring GE and intrinsic component of the definition of the syndrome is available in only a few centres around the world. Thus, true population-based epidemiological data are not available. An attempt was made to calculate epidemiological data by extrapolating results obtained from 450 patients who underwent scintigraphic GE studies and completed a symptom questionnaire at the Mayo Clinic, into those obtained from Olmsted County residents who received a modified version of the same questionnaire by mail.7 Notably, the adopted questionnaires had been previously validated, but did not fit exactly with the definition of gastroparesis, since neither included any question on postprandial fullness. Estimated prevalence in community subjects turned out to be 1.8%, a significantly higher figure than that of the prevalence of truly diagnosed gastroparesis of about 0.02%,27 thus suggesting a substantial proportion of the condition remaining unrecognised. Despite its limitations in both symptom features and GE measurement, the concept of gastroparesis is gaining momentum, due to an increased interest in the USA, where it represents an important precipitant cause of hospitalisations. These more than doubled from 1995 to 2004, exceeding those for GORD, gastric ulcers, gastritis and nausea/vomiting.28 The reasons for this phenomenon, which does not seem to be present in other parts of the world, where these diseases are generally treated in outpatients clinics, are not totally clear. One potential contributor may be the use of short GE tests, with assessment of gastric retention only at 90 or 120 min, which may lead to an erroneous increase in diagnoses of gastroparesis.29
Concept and implications
As outlined above, gastroparesis is defined by the presence of delayed GE in association with a variably defined set of symptoms, in the absence of a mechanical obstruction.2–4 ,6 ,7 ,10–14 The definition implicates, either directly or indirectly, that the delay in GE explains the symptom complex, i.e. is causally linked to the pattern and severity of specific symptoms (figure 1). Furthermore, enhancing or restoring the GE rate is considered the therapeutic target to obtain symptom relief, and this is reflected by the class name of ‘prokinetics’ for drugs that promote GE. As summarised in the next paragraphs, many of these implications are currently not achieved.
Key assumptions underlying traditional concepts of the role of delayed emptying in symptom generation and treatment response in gastroparesis.
Problem areas
Symptomatic gastroparesis or dyspepsia with delayed GE?
The definition of symptomatic gastroparesis largely overlaps with that of functional dyspepsia (FD) with associated GE delay. Dyspepsia is defined by the presence of symptoms believed to originate from the gastroduodenal region.29 FD is defined when these symptoms cannot be explained by any organic, systemic or metabolic diseases. Specifically, the Rome III criteria for FD30 are as follows: patients must have had one or more of the following symptoms for the past 3 months, with symptom onset at least 6 months prior to diagnosis: postprandial fullness, early satiety, epigastric pain or burning, as well as no evidence of structural disease that is likely to explain symptoms (including any condition detected by upper endoscopy). FD is further subclassified as: (a) postprandial distress syndrome (PDS) characterised by meal-induced fullness and satiety; (b) epigastric pain syndrome (EPS) by epigastric pain or burning. Moreover, a large patient group shows overlapping PDS and EPS symptoms. According to the Rome Criteria, FD should not be diagnosed in patients predominantly complaining of symptoms suggestive of GORD and/or IBS. Although apparently straightforward, this definition has raised remarkable disagreement,31 mainly because of its as yet ill-defined underlying pathophysiological mechanisms and unsatisfactory response to available treatments. Strictly associated to these uncertainties in the very definition of dyspepsia its epidemiological data vary quite extensively.32 However, more recent epidemiological data indicate that, after exclusion of GORD and IBS, the prevalence of dyspepsia in the general population ranges between 15% and 20% and the prevalence of FD between 10% and 15%, with PDS being the most numerous sub-subgroup.32 Due to these largely overlapping definitions, it is not surprising that almost the totality of patients with gastroparesis fall within the Rome definition of PDS.3 ,4 The two syndromes are even more difficult to be told apart, since, as previously mentioned, gastroparesis severity is defined not, as one would expect, by the degree of delay of GE, but rather by the intensity of dyspeptic symptoms.13
Symptoms associated with gastroparesis
Several studies evaluated the association of symptom pattern and severity with the presence of delayed emptying in FD. While smaller studies failed to find any association, larger scale studies inconsistently reported associations of delayed emptying with the severity of postprandial fullness, nausea and vomiting (table 1).1 ,5 ,33–42 However, the difference in symptom pattern between those with normal and with delayed emptying remained small, and symptom pattern was not able to predict the presence of delayed emptying. The issue of symptom association was further confounded by recent publications indicating that epigastric pain occurs frequently in gastroparesis and should be considered part of the symptom spectrum. On the other hand, no correlation between GE rate and pain ratings was found.43 The use of narcotic analgesics, which inhibit GE rate and which may be a treatment for chronic abdominal pain syndromes on one hand, but may also induce chronic abdominal pain on the other hand,44 is a major confounding factor. In the NIDDK consortium, more than 40% of patients were on narcotic analgesics, which really precludes analysing any association between symptoms of pain and delayed GE.3 ,4
Association between delayed gastric emptying and the symptom pattern in patients presenting with functional dyspepsia symptoms
Some studies also addressed the association of symptom pattern with the severity of the delay in emptying in gastroparesis patient cohorts. In 58 patients with idiopathic gastroparesis, GE rate was not correlated to the symptom pattern.15 In a study of 43 patients with diabetic gastroparesis and 114 patients with idiopathic gastroparesis from a single institution, the severity of nausea was weakly, but statistically significantly (R=0.17, p=0.035), correlated with gastric retention at 4 h.45 The most recent addition to these data are the results of the NIDDK Gastroparesis Clinical Research Consortium Registry in the USA. In a cohort of 243 patients with idiopathic gastroparesis, severely delayed emptying (% gastric retention at 4 h >35%) was associated with more severe symptoms of vomiting and loss of appetite.3 However, the GCSI8 was only borderline significantly associated with severe gastric retention.3
In diabetic gastroparesis, the link between delayed GE and symptom pattern was less extensively studied (table 2).46–50 In an early study in 87 diabetic patients, the overall severity score for gastric symptoms was statistically significantly correlated to the GE rate, but the correlation was numerically poor.47 In a more recent study from the same group, the severity of abdominal bloating/fullness, as well as female sex, mean blood glucose and body mass index were independent predictors of the presence of delayed emptying.48 The severity of the delay in GE rate was associated with increasing symptom severity, but the correlation was poor.48 Other studies, however, found no relationship between delayed emptying and symptom pattern in diabetes.50 In the largest study to date, GE rate and symptom pattern were studied in 247 patients with type 1 diabetes, no correlation was found between delayed emptying and symptom pattern or severity.41 A recent clinical trial, evaluating the effect of the ghrelin agonist TZP-102 in 201 patients with diabetic gastroparesis, found no correlations between GE rate and a daily diary at baseline or at the end of a 12-week treatment.51
Association between delayed gastric emptying and the symptom pattern in diabetic patients
One inherent problem in studying the association of delayed emptying and symptom pattern is the (lack of) consistency or variability of emptying and symptom pattern over time. Few studies assessed the reproducibility of delayed emptying over time. In a long-term follow-up study in patients with diabetic gastroparesis, symptoms persisted during a 12-year follow-up period, and so did mean GE rates, but the individual variability was high.52 In a 4-week trial with mitemcinal in gastroparesis, GE normalised in 26% of placebo-treated patients, and was especially high in the idiopathic group, reaching 38%.53 In idiopathic gastroparesis, a large placebo-controlled study with tegaserod showed that 50% of patients with significantly delayed GE normalised after 8 weeks of placebo therapy.54 A smaller study in patients with FD showed that GE rate and gastroparesis were less reproducible than the meal-related symptom pattern.55
One way to try and overcome the impact of variability in GE on the association with the symptom pattern is to measure symptoms at the same time as evaluation of GE. Two studies, evaluated symptoms over time during a GE study. The first study, in 218 patients with FD, found significantly higher scores for meal-induced fullness, bloating, pain and nausea in those with delayed emptying (n=44).56 The second, in 388 patients, also found higher severities of fullness, bloating and abdominal pain in those with delayed emptying (n=156).57
Prokinetic treatment in gastroparesis
A recent systematic analysis, incorporating data of prokinetic therapy trials in idiopathic and diabetic gastroparesis, failed to found a significant association between the improvement in GE rate and symptomatic benefit.58 Furthermore, a post-hoc analysis of studies with mitemcinal in diabetic gastroparesis showed that the greatest symptomatic benefit was paradoxically found in diabetics with normal GE.59 In the recent clinical trial of TZP-102 in 201 patients with diabetic gastroparesis, changes in GE rate and symptom improvement after 12 weeks were also not correlated.51 These findings show that the strength with which prokinetic drugs enhance GE does not predict the symptomatic benefit from the treatment. Furthermore, the mitemcinal studies suggest that the presence of gastroparesis may not even be a predictor of symptomatic benefit from prokinetic therapy.
Other pathophysiological mechanisms in patients with gastroparesis
In a gastric barostat study in 58 patients with idiopathic gastroparesis, high prevalence of impaired accommodation (43%) and hypersensitivity to gastric distention (29%) were found. GE rate was not correlated to the symptom pattern, but gastric accommodation and sensitivity to gastric distention were correlated with symptoms of early satiety, and gastric sensitivity was also correlated with the severity of epigastric pain.15 A gastric barostat study in 18 patients with diabetic gastroparesis also found a high prevalence of impaired accommodation and hypersensitivity to gastric distention, but no significant correlation with the symptom pattern was found.60 These studies suggest that in most patients with gastroparesis other pathophysiological abnormalities (hypersensitivity, impaired accommodation) co-exist, which may be at least as relevant for symptom generation as delayed emptying. Historically, GE testing was the first gastric function test to receive wide-spread use,61 which may have promoted creation of a separate diagnostic category, while gastric sensitivity and gastric accommodation testing are of more recent date, require invasive techniques and have never become widely used.62 ,63
Approaches to a more specific definition of gastroparesis
Higher threshold of GE delay for the definition of gastroparesis
FD is an extremely common condition, and up to one third of these have delayed GE when tested, but this is most often a mild delay, with relatively modest deviation from the normal range.1 ,6 ,33–42 Thus, it has been proposed to set a higher standard by using an arbitrary higher cut-off for gastroparesis than for delayed emptying.64 However, in one study using this approach, the demographic properties and symptom pattern were very similar in those with delayed emptying (outside the mean±2 SD range in healthy controls) or with gastroparesis (arbitrarily outside the mean±3 SD range).64 The available literature shows that the finding of delayed GE is often not stable over time.52 ,54 ,55 Hence, it has also been proposed to use the term ‘gastroparesis’ only when at least two emptying tests had been performed which showed delayed GE.55 However, the impact on symptom pattern and prevalence of this approach has not been formally tested.
Symptom-based approach
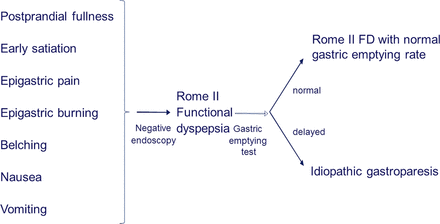
The Rome I and II consensus both defined dyspepsia as pain or discomfort centred in the upper abdomen, with discomfort including multiple symptoms such as postprandial fullness, upper abdominal bloating, early satiety, epigastric burning, belching, nausea and vomiting.65 ,66 Within this broad symptom complex, GE is one of a number of tests that can be done to elucidate the underlying pathophysiology. In this approach, idiopathic gastroparesis can also be considered a subfraction of PDS identified after additional testing, and poorly predictable from the symptom pattern (figure 2). Indeed, starting from a group of patients with gastroparesis, the NIDDK consortium reported major symptom overlap between gastroparesis and PDS.3 ,4 A similar situation is found in diabetics, where the prevalence of symptoms suggestive of dysmotility is very high,67 but only a subgroup of these have delayed emptying.68 The others have been referred to as ‘diabetic gastropathy’.69
Role of gastric emptying testing and definition of idiopathic gastroparesis according to the Rome II definition, in which a broad upper GI symptom pattern represents functional dyspepsia.
The most recent consensus, Rome III, aimed at a more homogeneous condition and defined dyspepsia as the presence of at least one of 4 symptoms (postprandial fullness, early satiation, referred to as PDS, and epigastric pain or epigastric burning, referred to as EPS) that are thought to originate from the gastroduodenal region.30 Symptoms of nausea and vomiting were no longer considered cardinal FD symptoms and were moved into separate categories of functional nausea and vomiting disorders.30 As several studies had shown association of delayed GE with symptoms of nausea and vomiting, it was proposed that idiopathic gastroparesis would be diagnosed as a further work-up of refractory nausea and vomiting symptoms, rather than FD symptoms70 (figure 3).
Role of gastric emptying testing and definition of idiopathic gastroparesis according to the Rome III definition, in which specific symptoms excluding nausea and vomiting represent functional dyspepsia.
The lack of correlation between symptoms and delayed emptying in patients diagnosed with gastroparesis within a broad symptom complex has been a cause of confusion and of therapeutic perseverance and has failed to generate any therapeutic advance for these patients, with all of the recent treatment trials in gastroparesis failing to achieve meaningful symptom control.51 ,53 ,55 ,59 ,71 The Rome III approach is attractive, as it focuses on the initial symptom pattern to distinguish between FD and (idiopathic) gastroparesis, based on symptoms that were shown to be associated with delayed GE and has the potential to resolve a long-lasting debate. According to this concept, epigastric pain should also not be included as a cardinal symptom in gastroparesis, as it has never been shown to correlate to the presence or severity of delayed GE. However, to date, this symptom-based approach will need further evaluation and validation in large pathophysiological, epidemiological or treatment intervention studies. It remains to be evaluated whether this approach will be able to classify all patients and whether it will lead to improved management and symptom control. A recent analysis of patients with nausea and vomiting enrolled in the NIDDK gastroparesis consortium described a subgroup of patients with normal emptying, but their symptom pattern did not differ from those with delayed emptying. Moreover, not all patients in this cohort fulfilled the Rome criteria of chronic nausea and vomiting disorders, and there was major overlap with FD according to Rome criteria.72
Conclusions
From a semantic standpoint dyspepsia and gastroparesis are two separate entities that should not be confounded. The word dyspepsia derives from the Greek word dus=bad and pepto=digestion (i.e. impaired digestion) and indicates sensations referred by affected individuals, while gastroparesis derives from the Greek words gastro=stomach and pa’ resis=incomplete paralysis (i.e. partial paralysis of the stomach affecting motion, but not necessarily sensation), thus referring to a measurable impaired motor function of the stomach. The two terms are used in the current literature to embrace clinical conditions more complex than what strictly indicated by the stringent terminology.
Both terminologies have advantages and disadvantages. The main advantage of focussing on gastroparesis is that it drives attention on objective evidence of a measurable functional abnormality, thus providing a biomarker to identify the condition. With GE being the first and most widely available test, there is an obvious focus on diagnosing abnormalities of GE, which led to the concept of ‘gastroparesis’. However, this terminology implicates that the clinical features of affected individuals are associated with the pathophysiological abnormality and this is not invariably true, since patients with normal or even accelerated GE can present with identical clinical manifestations, while subjects with (even markedly) delayed GE can be asymptomatic.73 Furthermore, there is a risk that patients with similar clinical features, but different underlying pathophysiological mechanisms and normal GE, or even in the absence of any objective evaluation of GE are erroneously included in the diagnosis or, on the other hand, totally overlooked. Furthermore, clinging to the diagnosis of gastroparesis in patients whose symptoms do not respond to prokinetics may lead to advanced therapies like the use of potentially dangerous drugs, botulinum toxin, gastric electrical stimulation, percutaneous feeding tubes, surgery, etc.2 ,10 ,11 ,13 ,15 These interventions lack proven efficacy and have the potential to inflict considerable physical and psychosocial morbidity.
FD, on the other hand, is a vague term whose obvious disadvantage is that it is based exclusively on perceptions of unpleasant sensations reported by patients either into self-administered questionnaires or, more frequently, to their doctors who, in turn, must interpret their wording and formulate a diagnosis: a very soft ground. The main advantage of using the definition of dyspepsia is that it keeps the field open to investigating any possible biomarker and its real relationship with the clinical manifestations, without any preconstituted bias. Although less attractive in the short term, the latter definition bears promises of more thorough understanding of the mechanisms responsible for all dyspeptic symptoms. Thus, also from an intellectual point of view the two terms indicate two very different approaches to groups of patients that virtually overlap on clinical grounds. The debate is destined to stay, at least in the near future, bearing important clinical implications. For instance, with current definitions, if a drug is approved for gastroparesis only a minority of symptomatic patients with scintigraphically demonstrated delayed GE will have access to the treatment, thus leading to a major pressure on the few centres where these techniques are available, while drugs approved for EPS or PDS could be accessible to patients presenting with clinical manifestations regardless of potential underlying pathophysiological mechanisms.74
References
Footnotes
-
Contributors Both authors contributed to the contents of the article.
-
Funding JT is supported by a Methusalem grant from Leuven University.
-
Competing interests JT has given Scientific advice to AlfaWassermann, GlaxoSmithKline, Janssen, Rhythm, Shire, Takeda and Tsumura. VS has given advice to AlfaWasserman Pharma.
-
Provenance and peer review Commissioned; externally peer reviewed.