Article Text
Abstract
Evolution of treatment targets in IBD has increased the need for objective monitoring of disease activity to guide therapeutic strategy. Although mucosal healing is the current target of therapy in IBD, endoscopy is invasive, expensive and unappealing to patients. GI ultrasound (GIUS) represents a non-invasive modality to assess disease activity in IBD. It is accurate, cost-effective and reproducible. GIUS can be performed at the point of care without specific patient preparation so as to facilitate clinical decision-making. As compared with ileocolonoscopy and other imaging modalities (CT and MRI), GIUS is accurate in diagnosing IBD, detecting complications of disease including fistulae, strictures and abscesses, monitoring disease activity and detecting postoperative disease recurrence. International groups increasingly recognise GIUS as a valuable tool with paradigm-changing application in the management of IBD; however, uptake outside parts of continental Europe has been slow and GIUS is underused in many countries. The aim of this review is to present a pragmatic guide to the positioning of GIUS in IBD clinical practice, providing evidence for use, algorithms for integration into practice, training pathways and a strategic implementation framework.
- Inflammatory bowel disease
- gastrointestinal ultrasound
- mucosal healing
Statistics from Altmetric.com
Introduction
Therapeutic advances in the medical management of IBD have raised therapeutic expectations. A ‘treat-to-target’ approach has been proposed for IBD, wherein objective measures of disease activity are actively sought and used to guide subsequent management.1 The current target of therapy in IBD is attainment of mucosal healing, which has been shown to reduce rates of clinical relapse, hospitalisation and surgery.2–7
Endoscopic mucosal healing is a surrogate marker of intestinal healing in Crohn’s disease, which involves inflammation and structural disturbance of both the whole intestinal wall and the draining mesenteric lymph nodes. Endoscopic assessment of the mucosa is invasive, and has some, although small risk and is expensive, but has the potential benefit of being able to be performed by the treating clinician who can action the findings with contemporaneous changes to medical therapy. Yet the frequency by which colonoscopy should be performed according to the STRIDE recommendations is not compatible with many healthcare systems due to cost and accessibility.1 Such issues, coupled with the rising global incidence and prevalence of IBD, have placed a significant emphasis on imaging alongside endoscopy as a tool to diagnose and monitor intestinal inflammation.8–10 CT and MRI are by far the most commonly employed imaging modalities for IBD disease assessment in many countries. CT imaging is associated with ionising radiation exposure and is an inappropriate modality for serial monitoring, especially in the young where risk of carcinogenesis is substantial.11 MRI is costly, time-consuming and access can be difficult.12 The intravenous contrast agents used for both CT and MRI carry risks of acute kidney injury.13 14
GI ultrasound (GIUS), often performed by gastroenterologists as a point-of-care examination, is a cost-effective, non-invasive, radiation-free and easily accessible imaging modality, and allows transmural assessment of the bowel wall.15 16 However, uptake of GIUS in many countries outside continental Europe and incorporation into clinical trials has been slow, and it is often perceived to have limited clinical utility due to operator-dependence.17–19 Every diagnostic technique is subject to a degree of subjectivity and operator-dependence, and this criticism is perhaps more reflective of a previous lack of identifiable international performance and training standards in GIUS.20 Although data on accuracy and the relative pros and cons of GIUS in IBD as compared with other imaging modalities and endoscopy have been extensively reviewed, guidelines for incorporation of GIUS into clinical practice are not widely available.17–19 21–25 Moreover, the evidence level for use of GIUS alongside other imaging modalities and endoscopy in modern IBD management needs to be illustrated across a broad variety of clinical settings.
The aim of this review article is to provide a pragmatic approach to the positioning of GIUS in IBD clinical practice, so as to establish GIUS as a novel and useful tool in IBD management in countries where it is underused. Evidence levels are provided according to the Oxford Centre for Evidence-based Medicine, alongside clinical algorithms, and training pathways for GIUS.26
What is GIUS?
Equipment
GIUS involves the use of standard ultrasound equipment that is readily available in most hospitals. The minimum requirement is an ultrasound unit coupled with a low frequency (1–6 MHz) and a high frequency (2–12 MHz) transducer.25 Although portable ultrasound units are appealing, diminished resolution continues to limit their application in routine use.
Basic technique
GIUS is undertaken using a transabdominal approach. Patient preparation is not generally required and the examination typically takes between 5 and 20 min (depending on the question being addressed). The ultrasound transducer is applied to the abdominal wall, with gel used as an acoustic conductor. Standard two-dimensional brightness (B) mode is typically used. A low frequency transducer is initially used to elucidate gross anatomy at a deeper level, and a high frequency transducer is subsequently used for a detailed interrogation of the bowel wall. A systematic approach is employed to examine the whole intestine, beginning in the left lower quadrant, with identification of the proximal rectum and sigmoid colon with progression proximally to evaluate all components of the large bowel. Identification of the ileocaecal valve and terminal ileum follows, with sweeping of all four quadrants for complete examination of the small bowel. Focused examination aims to identify both luminal and extraintestinal pathology including mesenteric lymphadenopathy and inflammatory fat, as well as complications such as fistulae, abscesses and visceral pathology. Identifiable abnormalities of the bowel include bowel wall thickening, preservation or loss of echostratification, strictures (luminal narrowing), fistulae and bowel dilatation (figure 1). Colour Doppler ultrasound optimised to detect blood flow within the bowel wall is routinely implemented to identify hypervascularity suggestive of active inflammation. Where a specific clinical question has been posed, a targeted examination may be performed. A systematic descriptive report should summarise the technique employed, the quality of the examination and detail the pertinent positive and negative intestinal features and extraintestinal findings.20 A detailed description of basic GIUS technique has been published by the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB).25
Large bowel inflammation detected using GI ultrasound. (A) Normal sigmoid colon with a wall thickness of 1.8 mm. (B) Active inflammation within the sigmoid colon characterised by increased wall thickness (5.2 mm), partial loss of wall stratification and increased hyperaemia on colour Doppler ultrasound. 1. Mesenteric hyperechogenicity associated with fibrofatty proliferation.
Special techniques
Small intestine contrast ultrasound
Small intestine contrast ultrasonography (SICUS) involves examination of the small bowel following ingestion of a neutral contrast agent (typically 200–500 mL of a polyethylene-glycol solution). SICUS is highly accurate in detecting small bowel Crohn’s disease-related inflammation, as well as stricturing and penetrating complications. SICUS increases trainee accuracy in identifying small bowel pathology and improves the detection of proximal small bowel lesions in Crohn’s disease.27–29 The primary disadvantage of SICUS is the necessity for patient preparation, which limits its application as a point-of-care tool.
Transperineal ultrasound
Transperineal ultrasound involves detailed examination of the perineum using a small high-frequency curvilinear or linear transducer, and compared with endoanal ultrasound is less invasive and better tolerated by patients. Transperineal ultrasound is accurate in detecting and classifying perianal fistulising disease, as well as detecting perianal abscesses. Importantly, the transducers used for assessment of the transperineal ultrasound are the same transducers used for evaluation of the intestinal tract.30–32
Contrast-enhanced ultrasound
Contrast-enhanced ultrasound (CEUS) involves the use of an intravenous contrast agent, typically containing sulfur hexafluoride microbubbles. CEUS is helpful in characterisation of suspected abscesses and inflammatory phlegmons, confirming and tracking the route of a fistula and may help to distinguish between fibrotic and inflammatory stricturing disease.33 CEUS may also be helpful in quantitatively determining disease activity in IBD.34–36
Elastography
Ultrasound elastography provides a measure of the stiffness of tissue, representing a novel tool that may help in delineating between inflammatory and fibrotic components of intestinal strictures.37
How to position GIUS in IBD clinical practice?
GIUS does not supersede other imaging modalities or endoscopy in IBD, but is instead complementary. GIUS has unique properties that make it particularly useful in the management of IBD, as it represents a non-invasive, cost-effective and readily repeatable test that may be performed at the point of care (table 1). Moreover, GIUS provides an objective assessment of inflammation in IBD, imperative to clinical decision-making in the ‘treat-to-target’ era.1 23 38
Current and emerging roles for GI ultrasound in IBD
Where to perform GIUS and by whom?
Point-of-care ultrasound versus stand-alone GIUS list?
In the clinical setting, GIUS can be considered an ‘extension of the examining hand’, gleaning considerably more information than physical examination, while incurring minimal additional time, cost or patient inconvenience.20 Point-of-care ultrasound (PoCUS) is increasingly being used in emergency and critical care settings, as well as for diagnosis and monitoring of other chronic inflammatory conditions.39 40
GIUS holds advantages over other imaging modalities and endoscopy in that it can be performed in real-time at the bedside, thereby expediting diagnosis, improving allocation of resources and facilitating earlier initiation of therapy.16 GIUS as a preliminary investigation at the point of care has been shown to accurately discriminate between inflammatory and non-inflammatory pathology in patients with abdominal pain and diarrhoea, as compared with ileocolonoscopy as the reference standard (sensitivity 80%–90%, specificity 94%–97.8%).16 41 42 GIUS performed in clinic can also make a significant impact on clinical decision-making in routine IBD care, altering the management strategy in patients with Crohn’s disease in up to 60% of cases.15 In addition, GIUS is an ideal modality in the paediatric population, given the potential involvement of and direct observation by parents, with ample teaching opportunity.43 In summary, PoCUS represents a promising tool to optimise and expedite management of IBD (figure 2).
Algorithm for point-of-care ultrasound (PoCUS).
Beyond PoCUS, many centres have stand-alone lists dedicated to GIUS for patients with IBD. Stand-alone GIUS lists may be advantageous in expanding capacity to perform GIUS, particularly for gastroenterologists who are themselves not trained in GIUS, as well as maximising healthcare resource allocation via predictable patient bookings and session utilisation. The workflow model is ideally temporally associated with clinician assessment so the pertinent information can be applied clinically.
GIUS performed by gastroenterologist or radiologist?
The capacity for physicians to perform focused ultrasound examinations relevant to their field of specialty is gaining acceptability, particularly in acute and emergency medicine.44 Physician-performed ultrasound allows acceptable diagnostic accuracy in the clinical context, without the necessity for formal training in radiology nor a full diagnostic knowledge and expertise in broader ultrasonography.20 The benefits of ultrasound performed by a member of the team delivering IBD care are numerous, including a deep understanding of the clinical question being posed, as well as the capacity for interpretation of findings in the clinical context, and discussing and expediting management decisions in real-time.15 Physician-performed ultrasound has also been reported to strengthen rapport between doctors and patients in other chronic disease settings.20 45
Physician-performed GIUS is yet to gain universal acceptance, and some guidelines categorise GIUS as a specialist skill within the broader context of abdominal sonography.20 The benefit of GIUS being performed within a radiology department by a dedicated sonographer is the potential for increased diagnostic accuracy in detecting pathology beyond the bowel with interpretation by a trained radiologist. Established radiology reporting software allows ready archiving of images and dissemination of findings within hospital networks. Although such connectivity is not always available within gastroenterology departments, this can be overcome through collaboration and integration with existing radiology networks. Experience in GIUS within radiology departments is variable, and the necessity for referral to radiology for GIUS and a further appointment time detracts from the potential for PoCUS and limits real-time decision-making. Collaboration with diagnostic imaging experts is important, particularly where anatomy is complex from prior surgery, or complications have altered the normal structures, emergent CT or MR may be invaluable.
Where to perform GI ultrasound (GIUS) and by whom?
Maximal benefit of GIUS is derived from point-of-care ultrasound performed by a member of the gastroenterology team delivering IBD care (evidence level 5).
GIUS performed in a stand-alone list, either by a gastroenterologist or within the radiology department, may be useful to increase capacity to perform the test beyond point-of-care ultrasound (evidence level 5).
GIUS for the diagnosis of IBD
The gold standard for the diagnosis of IBD is not well defined. Current guidelines suggest the diagnosis of IBD is based on a composite evaluation of clinical symptoms, endoscopy, histology and imaging.46 47 Multiple studies have compared GIUS with a reference standard of ileocolonoscopy or magnetic resonance enterography (MRE).48 The most sensitive sonographic measure for the diagnosis of IBD is increased bowel wall thickness.17 18 48 Much like the other established cross-sectional imagine modalities CT and MR, other markers of intestinal inflammation contribute to the diagnosis of IBD, including abrogation of bowel wall stratification, increased vascularity on Doppler ultrasound, extraintestinal features including mesenteric hyperechogenicity, lymphadenopathy and the presence of complications (stricturing or penetrating disease).17 18
GIUS has been predominantly evaluated as a diagnostic tool for Crohn’s disease. In a study of 249 patients with suspected Crohn’s disease, GIUS demonstrated sensitivity of 94%, specificity of 97%, positive predictive value of 97% and a negative predictive value of 94% in ascertaining the diagnosis of Crohn’s disease.49 Overall, data from systematic reviews and meta-analyses report sensitivity and specificity for diagnosis of Crohn’s disease between 75%–90% and 75%–100%, respectively, with an area under the receiver operating curve (AUROC) of 0.94, consistent with good diagnostic accuracy.17 18 21–24 The diagnostic performance for ultrasound is higher for disease located in the ileum and sigmoid/descending colon, as compared with the rectum, duodenum and proximal jejunum.28 41 In the setting of a high pretest probability, GIUS represents a cost-effective tool to establish a diagnosis of Crohn’s disease and help guide and expedite the need for further diagnostic investigations including ileocolonoscopy.12 Ultrasound is accepted as the first-line tool for the primary diagnosis of Crohn’s disease in the paediatric setting, where a non-invasive strategy that does not require sedation is preferable.17
GI ultrasound (GIUS) for the diagnosis of IBD
Crohn’s disease
GIUS is a useful non-invasive technique for initial evaluation of patients with suspected Crohn’s disease, and findings correlate well with endoscopy and cross-sectional imaging (evidence level 2b).
UC
GIUS is useful for initial evaluation of patients with suspected UC to detect the presence and extent of colonic inflammation, although views of the rectum are limited (evidence level 3b).
The role of GIUS in diagnosing UC is less compelling given the ability for sigmoidoscopy and histology to readily establish this diagnosis in the clinical context and the restriction of the pathology to the mucosa.50 Nonetheless, GIUS has a good capacity to interrogate left and right colonic inflammation, although views of the rectum may be limited.41 The reported sensitivity and specificity of GIUS in the diagnosis of colonic inflammation in UC are 90% and 96%, respectively on a per-patient analysis and 74% and 93% on a per-segment analysis.21 51 GIUS for initial evaluation of suspected UC is also informative as to disease extent, which may not be elucidated by flexible sigmoidoscopy alone.50
GIUS for assessment of IBD disease activity, extent and bowel damage
IBD disease activity
Systematic reviews have reported GIUS to be accurate in the assessment of Crohn’s disease activity of the ileum and colon when compared with both clinical activity scores and ileocolonoscopy, with an overall sensitivity and specificity of 85% and 91%, respectively.18 23
There are several markers of intestinal inflammation. First, bowel wall thickness is the most commonly used and reliable measure of IBD disease activity, although relatively poor interobserver agreement results from lack of international agreement as to standard measurement technique and cut-off values for inflammation.48 52 The sensitivity of detecting bowel inflammation is higher if a cut-off value of 3 mm is used for bowel wall thickness, whereas a higher specificity is associated with a cut-off of 4 mm (sensitivity 88%, 75%; specificity 93%, 97%, respectively).53 Second, vascularity of the bowel wall as assessed using Doppler ultrasound has also been used as a measure of intestinal inflammation in Crohn’s disease, although quantification has not been standardised.34 54 There is minimal intramural blood flow detectable using Doppler ultrasound in normal bowel wall. However, in the setting of inflammation, an increase in both intramural and extramural blood flow may be detected.55 Third, mesenteric hypertrophy resulting from fibrofatty proliferation (‘creeping-fat’) in the setting of transmural inflammation is detected as hyperechoic signals adjacent to inflamed bowel using GIUS.48 Fourth, mesenteric lymphadenopathy is a non-specific marker of inflammation, although may persist beyond resolution of active intestinal inflammation and lacks specificity for IBD. Fifth, normal bowel peristalsis is usually altered or absent in an inflamed bowel segment, although standardisation of this parameter is challenging given subjectivity.56 Lastly, special techniques such as CEUS may also improve detection of active Crohn’s disease by allowing better sonographic visualisation of increased vascularity associated with inflammation; meta-analysis data report a sensitivity of CEUS for active Crohn’s disease of 93% and an AUROC of 0.96.57
There are several scoring systems for disease activity assessment using GIUS in Crohn’s disease, although until recently, none has been validated.58–60 The Simple Sonographic Score is a validated index incorporating bowel wall thickness and colour Doppler ultrasound, and this has demonstrated 92% sensitivity and 82% specificity compared with endoscopy.61 The most widely used scoring system is the Limberg Score, incorporating bowel wall thickness and Doppler vascularity (table 2).55 62
GI ultrasound scoring system proposed for patients with Crohn’s disease
There are fewer available data on disease activity assessment using GIUS in UC, primarily due to its perceived lack of need due to the availability of and accessibility of disease to endoscopic evaluation. Submucosal hyperechoic thickening is frequently observed in active UC, although quantitative measures are poorly defined.48 As with Crohn’s disease, bowel wall thickness and intramural Doppler vascularity have been used to evaluate disease activity in UC.17 63 64 Sonographic identification of mucosal thickness of >1.5 mm, bowel wall thickness >4 mm, mucosal irregularity and absence of haustra have been shown to correlate well with clinical and endoscopic disease activity indices.65 66 Although there is no validated GIUS score of disease activity in UC, a scoring system proposed by Parente et al has been shown to be concordant with endoscopic inflammation in moderate-to-severe UC (weighted kappa 0.76–0.90).63 64
IBD disease extent
GIUS has been shown to be accurate in detecting the extent of small bowel Crohn’s disease as compared with radiology and surgery, with a pooled sensitivity of 86% (95% CI 83% to 88%) and specificity of 94% (95% CI 93% to 95%).17 18 23 28 41 49 A notable caveat is that GIUS seems to be less sensitive than MRE in establishing the presence and extent of proximal small bowel Crohn’s disease.49 GIUS is of benefit in establishing the disease extent of Crohn’s disease beyond the reach of the colonoscope, yielding further information (including the presence of complications) in more than a third of patients with inconclusive colonoscopy.67
The accuracy of GIUS in evaluating small bowel inflammation in Crohn’s disease may be enhanced with the use of SICUS, where oral contrast ingestion leads to luminal distension with fluid, reducing bowel gas artefact and improving the quality of bowel wall visualisation.28 29 68 69 The pooled sensitivity of detecting involvement of the jejunum and ileum in Crohn’s disease using SICUS is 98.7% (95% CI 95.2% to 100%) and 97.4% (95% CI 95% to 99.8%), respectively, with specificity of 100% for both.23
GIUS is accurate in detecting disease extent in UC proximal to the rectum.50 The combined reported sensitivity and specificity for detection of colonic inflammation is 74% and 93% per segment,21 41 50 whereas the sensitivity for detecting rectal inflammation is poor (14%) with good specificity (99%).41 GIUS may be particularly useful in establishing disease extent where full colonoscopy is inappropriate or unsafe, such as in the setting of acute severe UC.
Bowel damage
Assessment of disease severity of IBD has evolved beyond assessment of measurable inflammatory burden to evaluation of the disease course and associated cumulative structural bowel damage.70–72 GIUS is an accurate modality for assessing bowel damage in Crohn’s disease, correlating closely with MRE in calculation of the Lemman Index, using scoring criteria of previous surgery, disease location, extension and intestinal complications.73 The Sonographic Lesion Index for Crohn’s disease (SLIC) has been proposed as a numerical index to quantify small bowel damage in Crohn’s disease using SICUS.60 SLIC takes into account bowel wall thickness, lumen diameter, lesion length and number, along with the presence of complications, mesenteric changes and lymphadenopathy and has been shown to correlate with the need for bowel surgery within the subsequent 12 months.60
Although a discrete index for sonographic evaluation of bowel damage in UC has not been proposed, the presence of colonic structural damage may be identified using GIUS with detection of loss of haustral folds and postinflammatory polyps.41 65
GI ultrasound (GIUS) for assessment of IBD disease activity, extent and bowel damage
IBD disease activity
GIUS is an accurate modality for assessment of IBD disease activity, although a validated scoring system does not exist (evidence level 2b).
IBD disease extent
GIUS is accurate in determining IBD disease extent, although assessment of the proximal small bowel and rectum are suboptimal (evidence level 2b).
Bowel damage
GIUS may be useful for evaluating cumulative bowel damage in Crohn’s disease, although evidence in UC is limited (evidence level 3b).
GIUS for detection of IBD complications
Strictures
GIUS is a highly accurate tool for the detection and assessment of small and large bowel strictures in Crohn’s disease (figure 3).17 Using surgical resection specimens as the gold standard, multiple studies have shown that GIUS performed well with a pooled sensitivity in detecting strictures of 79% (95% CI 71% to 84%) and specificity 92% (95% CI 87% to 96%).18 GIUS also compares favourably with other cross-sectional imaging modalities such as CT and MRI for stricture assessment.17 A significant advantage of GIUS over other cross-sectional imaging modalities is the capability to visualise intestinal motility in real-time, differentiating definite strictures from either functional contractions or collapsed bowel. SICUS has been proposed to have even better accuracy for the detection of small bowel strictures compared with standard GIUS as it eliminates small bowel gas by distending the bowel lumen with fluid.68 74
Small bowel stricture evaluation using GI ultrasound. (A) Normal terminal ileum with a patent lumen (horizontal arrow) and a normal wall thickness of 2.2 mm. (B) Inflammatory stricture within terminal ileum displaying a thickened bowel wall displaying a thickened bowel wall (1), narrowed lumen (2. thin gas line), proximal dilatation (3) and mesenteric hyperechogenicity (4).
GIUS can assist in determining the degree of inflammation versus fibrosis in a stenosed segment of bowel; such information has a significant impact on treatment strategy. Both loss of wall stratification and increased Doppler vascularity within a stricture are suggestive of predominantly inflammatory disease.54 62 75
CEUS is an accurate modality for the assessment of inflammation and fibrosis in strictures. Using dedicated computer software, quantitative analysis of time-to-peak intensity, area under the curve and curve intensity can be performed with higher values being consistent with inflammation.76–78 EFSUMB recommend routine use of CEUS for stricture assessment.79
Enteric fistulae
GIUS can be used to accurately assess complications of Crohn’s disease. Using standard brightness mode imaging, fistulae appear as a hypoechoeic region in direct communication with the affected segments of bowel. In a systematic review, the pooled sensitivity for GIUS in the detection of enteric fistulae was 74% (95% CI 67% to 79%) and specificity 95% (95% CI 91% to 97%) compared with surgery.18 GIUS has also been found to be of similar accuracy when compared with CT and MRI for this indication.80 81
CEUS can also be used as an adjunct to assess enteric fistula tracts. These tracts display as an anechoic (black) tract, while the remainder of the screen becomes bright as the intravenous contrast is taken up by surrounding tissue. This technique for fistula assessment is also recommended by EFSUMB.79
The drawback to GIUS for fistula assessment relates to difficulties visualising bowel deep within the pelvis. Therefore, if a clinician suspects the presence of an enteric fistula or fistula in the deep pelvis, cross-sectional imaging with MRI (or transvaginal GIUS for women) is recommended to ensure all bowel segments (especially those within the pelvis) are visualised.17
Intra-abdominal abscesses
GIUS with simple brightness mode imaging has a high degree of accuracy for the detection and monitoring of intra-abdominal abscesses. In a systematic review, GIUS was reported to have a pooled sensitivity of 84% and a specificity of 93% for abscess detection compared with surgery.18 The same limitations of GIUS for fistula assessment regarding the visualisation of deep pelvic structures apply for abscess detection.
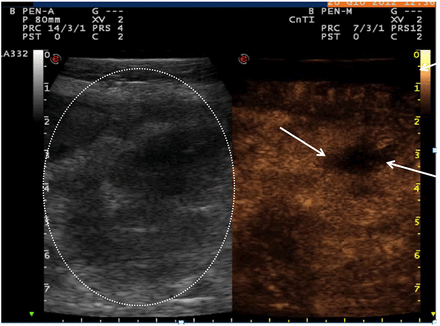
A clinically useful application for CEUS is in differentiating inflammatory phlegmons from intra-abdominal abscesses (figure 4). It can often be difficult for radiologists to distinguish between these two complications with cross-sectional imaging such as MRI or CT, yet the clinical importance of this delineation is crucial for management decision-making. An analysis of 57 inflammatory masses in 50 patients found CEUS had a sensitivity, specificity and accuracy of 97%, 100% and 98%, respectively, when compared with surgery, percutaneous intervention or MRI, with a high interobserver agreement (kappa=0.953).82 CEUS is advantageous compared with other imaging modalities in that it is a rapid test with images obtained within 2 min of administration of the intravenous contrast agent. Furthermore, as the sulfur hexafluoride microbubbles are cleared via the respiratory system, there is no concern regarding renal or hepatic impairment, as opposed to the contrast agents used for MRI and CT. CEUS may be useful is guiding therapeutic interventions such as abscess drainage.
Abscess assessment using GI ultrasound. Large inflammatory phlegmon in brightness (two-dimensional) mode ultrasound (circled greyscale image) alongside contrast-enhanced ultrasound image 45 s after administration of intravenous contrast highlighting a small abscess (arrowed hypoechoic area).
GI ultrasound (GIUS) for assessment of complications of IBD
Strictures
GIUS is a useful non-invasive technique for the assessment of bowel strictures, and findings correlate well with surgery, endoscopy and cross-sectional imaging (evidence level 2b).
Intra-abdominal fistulae
GIUS is an accurate technique for the detection of fistulae (evidence level 2b).
Intra-abdominal abscesses
GIUS is an accurate technique for the detection of abscesses (evidence level 2b).
CEUS is useful in distinguishing inflammatory phlegmons from abscesses (evidence level 3b).
GIUS for assessment of response to therapy and prognostication
The target of treatment in IBD has evolved from symptomatic improvement to resolution or improvement of objective markers of inflammation, which has led to an increased intensity of objective disease activity monitoring to inform therapeutic strategy.1 Current ‘treat-to-target’ guidelines recommend endoscopic evaluation of disease at 3-monthly intervals during the active phase of IBD and within 3–6 months of a change in therapy.1 A shorter interval between endoscopic evaluation of IBD has been shown to increase the likelihood of achieving mucosal healing.83 Such recommendations are aspirational rather than practical or patient-friendly, but they do stress the importance of regular objective assessment of intestinal inflammation and at relatively short intervals. There is emerging evidence that GIUS may be an alternative, better-tolerated and economically responsible modality for monitoring disease activity in response to therapy in IBD.
GIUS has been shown to be accurate in monitoring disease activity in response to therapy in Crohn’s disease as compared with clinical activity scores, biomarkers and ileocolonoscopy (figure 5). In a small group of patients with Crohn’s disease initiated on biological therapy, an ultrasound-based scoring system (SLIC) was shown to significantly correlate with clinical response to treatment.84 The large multicentre TRUST study evaluated ultrasound indices sequentially over 1 year after treatment-intensification for Crohn’s disease, finding a significant reduction in bowel wall thickness, fibrofatty proliferation and Doppler vascularity at 3 and 12 months compared with those at baseline (P<0.01 for all), and these correlated with reduction in C reactive protein (CRP) at 3 months (P≤0.01).85 Using ileocolonoscopy as the reference standard, normalisation of ultrasound indices closely correlates with mucosal healing in 30 patients with Crohn’s disease started on biological therapy (weighted kappa 0.73, P<0.001).86 However, given that inflammation in Crohn’s disease is transmural, it must be noted that endoscopic mucosal healing may not be equivalent to ‘transmural healing’ as assessed using ultrasound.87 In 66 patients with Crohn’s disease on maintenance biological therapy for 2 years or more, endoscopic mucosal healing was numerically more frequently achieved than sonographic transmural healing (38% vs 25%), although the difference was not statistically significant.87 Any prognostic value of achieving transmural healing beyond endoscopic mucosal healing has yet to be established.
Perianal fistula assessment using GI ultrasound. (A) Longitudinal view scan of the anal canal (a) and rectum (r) showing a small posterior intersphincteric abscess (anechoic area with asterisk). (B) Transverse view of the anal canal of the same patient showing the same posterior abscess (anechoic area with asterisk). Internal anal sphincters are indicated by the blue arrows and external anal sphincters are indicated by the red arrows. (C) Schema of transperineal ultrasound demonstrating saggital or longitudinal plane (transducer placed over anus in anteroposterior direction, with anterior side on the right) and transverse plane (transducer moved anteriorly and rotated by 90 degrees). Anal canal (a), rectum (r), internal anal sphincter (i), external anal sphincter (e).
Sonographic assessment of response to therapy has been shown to correlate with long-term clinical outcomes in Crohn’s disease. Using CEUS to assess response to therapy after initiation of corticosteroids or biological therapy in a small group of patients with Crohn’s disease, increased bowel perfusion at 1 month was associated with treatment failure within 12 months.88Among 51 patients with Crohn’s disease commenced on biological therapy, those who demonstrated sonographic improvement at 12 weeks were more likely to maintain sonographic response at 1 year.89 In this study, patients with a sonographic response at 1 year were less likely to require intensification or change of therapy or surgery within the subsequent 1 year than those without a sonographic response (11% vs 65%, respectively, P=0.0001).89
GIUS has been proposed as a surrogate for colonoscopy in the evaluation of response to therapy in UC if sonographic abnormalities were present during the active phase (figure 6).63 64 66 90 In patients receiving high-dose corticosteroids for moderate-to-severely active UC, a GIUS scoring system calculating bowel wall thickness and Doppler vascularity was found to be highly concordant with the endoscopic Baron score in assessing response to therapy over time (weighted kappa between 0.74 and 0.90).63 64 90 91 Moreover, persistence of disease activity on ultrasound at 3 months was associated with a high risk of endoscopic disease activity at 15 months (OR 9.1, 95% CI 2.5 to 33.5), illustrating the capacity of GIUS to prognosticate outcomes of disease.
Algorithm for non-invasive monitoring of disease activity in Crohn’s disease using GI ultrasound. *Corroborate with other non-invasive biomarkers such as faecal calprotectin. FGID, functional GI disorders.
It is widely acknowledged that symptoms are frequently discordant with active inflammation in IBD and that up to 60% of patients experience concurrent functional GI symptoms.92 93 GIUS can assist in delineation between active inflammation and other causes for symptoms in patients with IBD. The presence of symptoms without objective inflammation might lead, for example, to use of other efficacious therapies, such as bile salt sequestrants or a low FODMAP diet and avoid futile escalation of medical therapy with its associated costs and potential risks.94 95
GI ultrasound (GIUS) for assessment of response to IBD therapy
GIUS may be useful for monitoring response to therapy in IBD, in that it is responsive to change, and correlates with clinical and endoscopic response (evidence level 3b).
Improvement in GIUS parameters following a change in therapy may be useful to prognosticate risk of subsequent clinical relapse (evidence level 4).
In patients with IBD with ongoing symptoms despite medical therapy, GIUS can assist in delineating between ongoing active inflammation and other possible causes for symptoms (evidence level 5).
GIUS for assessment of postoperative Crohn’s disease recurrence
Approximately 70% of patients with Crohn’s disease will require intestinal resection at some time in their life and, of these, up to 70% require a second operation.96 Monitoring of Crohn’s disease activity postoperatively is important and allows for the optimisation of drug therapy to achieve disease control.97 Traditionally, monitoring has often been based on a combination of clinical assessment and biological markers of inflammation. However, there is poor concordance between these measures and endoscopic assessment (Rutgeerts’ score) for postoperative recurrence.97–99 Faecal calprotectin has reasonable accuracy in the diagnosis of postoperative recurrence of Crohn’s disease and it is superior to CRP and CDAI.99 Calprotectin measurement, however, has a moderate false-positive rate for the diagnosis of postoperative recurrence.99 Endoscopy, therefore, remains the gold standard for detecting postoperative recurrence and determining its severity.100
GIUS is a simple and non-invasive tool for the accurate diagnosis of postoperative endoscopic recurrence and has advantages over faecal calprotectin including the ability to document disease extent, strictures and the presence of extraluminal complications. GIUS is a sensitive test in detecting postsurgical clinical recurrence in Crohn’s disease with sensitivity and specificity between 82% and 100%, respectively.101 102 GIUS is also accurate in the diagnosis of endoscopic recurrence. A prospective study of 45 patients showed bowel wall thickness of >3 mm on GIUS had a sensitivity, specificity and positive and negative predictive values of 79%, 95%, 95% and 80%, respectively for endoscopic recurrence (Rutgeerts’ score ≥i2), and a sensitivity of 93% for severe postoperative recurrence (Rutgeerts’ score i3 or i4).103 In a study of 60 patients with previous ileocolonic resection, GIUS indices (wall thickness >3 mm and hypervascularity on colour Doppler ultrasound) had an accuracy of 88% for the diagnosis of endoscopic recurrence.104 Furthermore, bowel wall thickness of >5 mm or positive contrast enhancement improved accuracy further with sensitivity and specificity of 98% and 100%, respectively. The AUROC was 0.99, in high agreement with endoscopy (kappa=0.946).76
The timing of GIUS following intestinal resection remains unclear, although one group recommends testing as early as 3 months postoperatively, with colonoscopy reserved only for those with findings consistent with recurrent disease.105 The use of GIUS coupled with faecal calprotectin as a non-invasive approach in monitoring for postoperative Crohn’s disease recurrence is appealing and will be the subject of future studies.
GI ultrasound (GIUS) for postoperative Crohn’s disease
GIUS is a useful modality for monitoring for postoperative Crohn’s disease recurrence (evidence level 2b).
GIUS correlates well with ileocolonoscopy in detecting Crohn’s disease recurrence (evidence level 2b).
GIUS offers advantages over faecal calprotectin in its capacity to detect the extent of recurrence and the presence of strictures/complications (evidence level 5).
GIUS for assessment of perianal Crohn’s disease
Perianal Crohn’s disease is a common, complex and distressing complication of Crohn’s disease.106 107 Even with antitumour necrosis factor-α therapy, long-term healing rates are poor.108 Regular assessment with imaging is an important part of comprehensive patient care. MRI of the pelvis is the gold standard for imaging assessment of perianal disease. However, cost and timely access to MRI may limit frequent use. Endoanal ultrasound has also been described for the assessment of perianal disease, but is invasive, poorly tolerated if there is active perianal sepsis and impossible to perform if there is anal stenosis. Perineal views with this technique are incomplete and pathological changes expanding to the gluteal region cannot be assessed.
Transperineal ultrasound is a simple and generally painless technique to examine perianal pathology, and provides imaging of comparable quality to both endoanal ultrasound and MRI.109 110 Transperineal ultrasound has been investigated by several groups for the assessment of key aspects of perianal disease, including collections, fistulae and sinus tracts (figure 7).31 111–113 Transperineal ultrasound is accurate when compared with MRI with excellent agreement between transperineal ultrasound and MRI (kappa>0.83) for the detection of perianal lesions.30 114 115 A recent systematic review and meta-analysis confirms the accuracy of transperineal ultrasound in the assessment of perianal fistulae and abscesses.115
Algorithm for non-invasive monitoring of disease activity in UC beyond the rectum using GI ultrasound. *Corroborate with other non-invasive biomarkers such as faecal calprotectin. FGID, functional GI disorders.
GI ultrasound (GIUS) for perianal Crohn’s disease
Transperineal ultrasound is a useful non-invasive and painless technique to identify perianal fistulae and abscesses in Crohn’s disease (evidence level 2b).
Transperineal ultrasound is accurate in identifying perianal fistulae and abscesses using MRI as the reference standard (evidence level 2b).
Emerging and future roles of GIUS
Evaluation of stricturing disease
Elastography is emerging as a technology that may also provide further information regarding the degree of fibrosis in the setting of stricturing disease. Shear wave elastography (SWE) generates an acoustic pressure wave and the resultant shear wave speed through the bowel can be measured, correlating with increasing tissue density and stiffness.116 In small studies, SWE in combination with CEUS has been shown to accurately detect smooth muscle hypertrophy in the small bowel and differentiate between active and chronic bowel wall inflammation.116 Strain elastography measures the hardness of a tissue as a function of tissue compressibility and appears to correlate with the severity of ileal fibrosis in Crohn’s disease.37 117 118 These preliminary data suggest there may well be a role for elastography in the evaluation of IBD-related strictures, but further validation is required.
Application in functional GI disorders
Ultrasound allows real-time dynamic assessment of intestinal motility, yielding important functional information.119 Small studies show that GIUS may be helpful in identifying intestinal dysmotility in functional bowel disorders. Bowel contents may also be evaluated using GIUS, with the potential to allow identification of faecal loading without the need for abdominal X-ray. However, further research is needed to better define its use in clinical practice.120
Pregnancy
Assessment of IBD activity in pregnancy is challenging, given the risks associated with invasive endoscopic investigations and as well as ionising radiation and the uncertain safety profile of contrast agents such as gadolinium. GIUS represents a plausible non-invasive modality for disease activity assessment in pregnancy, although there is a paucity of data on the subject. Research is currently underway to determine the effect of the gravid uterus on accuracy of GIUS in pregnancy.
Evaluation of transmural healing in IBD
The current target of medical therapy in IBD is endoscopic mucosal healing or normalisation of the bowel mucosa as seen at colonoscopy.1 However, mucosal healing is only a surrogate marker for intestinal healing. Beyond mucosal healing, histological remission may be associated with improved outcomes.121 122 Furthermore, small studies have shown that normalisation of bowel wall thickness on MRE, or ‘transmural healing’, may be superior to endoscopic healing alone.123 GIUS holds promise as a useful tool to evaluate transmural healing in IBD longitudinally, although further studies are required to validate the prognostic significance of transmural healing before it can be considered a treatment target.
Future roles of GI ultrasound (GIUS)
Preliminary data suggest elastography can aid in differentiating inflammation from fibrosis in stricturing bowel disease (evidence level 4).
Functional bowel ultrasonography may be helpful in identifying intestinal dysmotility in functional bowel disorders (evidence level 4).
GIUS is conceptually a useful tool for IBD activity assessment in pregnancy, although data are lacking (evidence level 5).
GIUS may be a useful tool to evaluate transmural healing in IBD, the prognostic significance of which requires further validation (evidence level 5).
Limitations of GIUS in IBD
The utility of GIUS in the setting of obesity can be limited, where the depth of penetration may impede accuracy of imaging as well as the capacity for colour Doppler ultrasound.25 However, the sonographic properties of adipose tissue can vary widely between individuals and there is no defined body mass index above which GIUS is not recommended. Due to its position behind the urinary bladder within the pelvis, the sensitivity of GIUS for detecting rectal inflammation is poor.41 50 Similarly, GIUS lacks accuracy in identifying fistulising complications within the pelvis. GIUS is less accurate that other cross-sectional modalities in detecting proximal small bowel inflammation, particularly in the retroperitoneal duodenum.41 49 Although it is possible to detect advanced neoplastic lesions using GIUS, there is no evidence that GIUS is able to detect colitis-associated dysplasia. GIUS is therefore not recommended as a tool for surveillance in long-standing colitis.
Establishing GIUS in IBD practice
Learning GIUS
Until recently, one of the key challenges in facilitating the uptake of GIUS has been the lack of an internationally recognised training curriculum.20 A core curriculum coupled with identifiable training and performance standards in GIUS is fundamental to ensuring competency among physician sonographers.
Despite a prior lack of ultrasound experience, gastroenterologists are equipped with the knowledge and dexterity to rapidly acquire competency in GIUS.20 There is minimal difference in the learning curve for GIUS between those with and without prior ultrasound experience.20 There are multiple platforms for learning GIUS, including the use of models, for which there is broad evidence for improvement in skill and knowledge acquisition.124 Different clinical scenarios may be entered into these models to demonstrate salient sonographic features. e-Learning tools may also be useful for learning ultrasound skills, particularly when coupled with patient-based learning that allows exposure to a range of pathology and individual variance.20
The key learning domains of GIUS are a basic knowledge and conceptual framework of ultrasound, knowledge of intestinal anatomy, ultrasound examination technique and interpretation and reporting of findings.20 125 Gastroenterologist sonographers need to be able to recognise intestinal and extraintestinal abnormalities and should have the ability to interrogate findings. Interpretation of ultrasound findings in the clinical context to support clinical decisions through the provision of a clinically useful report are key components of GIUS.
Training pathways
The International Bowel Ultrasound Group (IBUS; www.ibus-group.org), consisting of an international group of GIUS experts, have proposed an education model for GIUS.126 This model represents the first internationally recognised curriculum for learning GIUS, comprising basic educational components (intensive theoretical and hands-on GIUS course), supervised clinical training at an accredited training centre (4 weeks with creation of a log-book of typically >200 GIUS cases performed) and a summative assessment prior to graduation (European Crohn’s and Colitis Organisation Congress GIUS Workshop).126
National implementation of GIUS
Despite ample evidence in the literature supporting GIUS in IBD, it has been underused in many countries outside of continental Europe.19 This has been historically due to a lack of local expertise and available training in the technique, coupled with scepticism as to its clinical utility and lack of fiscal reimbursement for physician-performed ultrasound.19 Establishing GIUS in clinical practice requires a co-ordinated approach; a suggested framework is shown in table 3.
A suggested national framework for establishing GI ultrasound (GIUS) in clinical practice
Conclusions
GIUS is a valuable non-invasive tool in the management of IBD that has the potential to shift clinical practice paradigms. GIUS complements conventional endoscopy and cross-sectional imaging, and can be performed at the point of care to expedite clinical decision-making and optimise patient management. Integrating a novel tool into established practice norms is challenging, and a co-ordinated approach at a national level is required, incorporating identifiable training and performance standards in GIUS, education and promotion of the role of GIUS in clinical practice and generation of local research evaluating the accuracy and utility of the technique to engender clinician confidence.
References
Footnotes
Contributors RVB, AF, EKW, JB, KT, AA and PRG provided substantial contribution to the conception and design of the work. All authors contributed to drafting and critical revision of the manuscript. The final version of the manuscript was approved by all coauthors.
Funding RVB has received speaker honoraria from AbbVie, Shire Australia, Janssen, Takeda Pharmaceuticals Australia. He received conference travel support from Ferring Australia and Takeda Pharmaceuticals Australia. He has received research and/or operational infrastructure support from Ferring Australia and Takeda Pharmaceuticals Australia. He has received funding from a GESA/Ferring IBD Clinician Establishment Award. AF has received speaker fees from AbbVie, Janssen–Cilag, Takeda Pharmaceuticals Australia, Shire Australia. He has received research funding for investigator-driven studies from AbbVie. He has received travel grants from Pfizer and Ferring. PRG has served as consultant or advisory board member for AbbVie, Ferring, Janssen, Merck Sharp & Dohme, Nestle Health Science, Danone, Allergan, Pfizer, Celgene and Takeda. His institution has received speaking honoraria from AbbVie, Janssen, Ferring, Takeda, Mylan, Danone and Pfizer. He has received research grants for investigator-driven studies from AbbVie, Janssen, Danone and A2 Milk Company. His department financially benefits from the sales of a digital application and booklets on the low FODMAP diet. He has published an educational/recipe book on diet. KT has served as an advisory board member for Merck Sharp & Dohme, has received speaker fees from AbbVie and travel grants from Aspen and Shire. GM has received speaker honoraria from AbbVie, Janssen, Takeda, Alfa Wasserman Poland and Italy, served as consultant or advisory board for Novartis, Alfa Wasserman, THD and Allergan. CM has served as an advisory board member for Janssen, MSD and Takeda, has received speaker fees from AbbVie, Falk Foundation, Ferring, Janssen, MSD, Shire, Takeda and financial support for clinical ultrasound studies from AbbVie. TK has served as consultant or advisory board member or received speaker fees from AbbVie, Biogen, Falk Foundation, Ferring, Jannsen, MSD, Mundipharma, Shire, Takeda, UCB as well as financial support for clinical ultrasound studies from AbbVie and Takeda. KLN has received consultative and speaker fees from AbbVie and Janssen Pharmaceuticals. She has also participated on advisory boards for Pfizer, AbbVie, Janssen Pharmaceuticals and Takeda Pharmaceutical. EKW has received consulting and speaker fees from AbbVie, Pfizer, Falk Foundation and Janssen. She has received research grants for investigator-driven studies from AbbVie and infrastructure support also from AbbVie. She has received funding from a GESA/Ferring IBD Clinican Establishment Award. JB has served as an advisory board member or consultant for AbbVie, Janssen, Takeda, Pfizer and Ferring Pharmaceuticals. He has received research grants from Ferring. He has received speaker honoraria from AbbVie, Janssen, Takeda, Pfizer, Shire and Ferring Pharmaceuticals. NSSA has received conference travel support from Napp Pharmaceuticals.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Correction notice This article has been corrected since it published Online First. The second and fourth author names have been updated as well as the legend for figure 6.