Article Text
Abstract
Objective The quality of colonoscopy is key for ensuring protection against colorectal cancer (CRC). We therefore aimed to elucidate the aetiology of postcolonoscopy CRCs (PCCRCs), and especially to identify preventable factors.
Methods We conducted a population-based study of all patients diagnosed with CRC in South-Limburg from 2001 to 2010 using colonoscopy and histopathology records and data from the Netherlands Cancer Registry. PCCRCs were defined as cancers diagnosed within 5 years after an index colonoscopy. According to location, CRCs were categorised into proximal or distal from the splenic flexure and, according to macroscopic aspect, into flat or protruded. Aetiological factors for PCCRCs were subdivided into procedure-related (missed lesions, inadequate examination/surveillance, incomplete resection) and biology-related (new cancers).
Results We included a total of 5107 patients with CRC, of whom 147 (2.9% of all patients, mean age 72.8 years, 55.1% men) had PCCRCs diagnosed on average 26 months after an index colonoscopy. Logistic regression analysis, adjusted for age and gender, showed that PCCRCs were significantly more often proximally located (OR 3.92, 95% CI 2.71 to 5.69), smaller in size (OR 0.78, 95% CI 0.70 to 0.87) and more often flat (OR 1.70, 95% CI 1.18 to 2.43) than prevalent CRCs. Of the PCCRCs, 57.8% were attributed to missed lesions, 19.8% to inadequate examination/surveillance and 8.8% to incomplete resection, while 13.6% were newly developed cancers.
Conclusions In our experience, 86.4% of all PCCRCs could be explained by procedural factors, especially missed lesions. Quality improvements in performance of colonoscopy, with special attention to the detection and resection of proximally located flat precursors, have the potential to prevent PCCRCs.
- Colorectal Cancer
- Colorectal Adenomas
Statistics from Altmetric.com
Significance of this study
What is already known about this subject?
-
Although colonoscopy protects against colorectal cancer (CRC), its effectiveness in the proximal colon lags behind.
-
Procedural factors and biological features may be responsible for the occurrence of postcolonoscopy CRCs (PCCRCs), yet their precise contribution remains unknown.
What are the new findings?
-
In our experience, 2.9% of all CRCs found were PCCRCs, diagnosed on average 26 months after an index colonoscopy.
-
The majority of PCCRCs can be explained by procedural factors, especially missed lesions (57.8%), inadequate examination/surveillance (19.8%) or incomplete polypectomy (8.8%).
-
PCCRCs were featured by proximal location, small size and a flat appearance.
How might it impact on clinical practice in the foreseeable future?
-
Quality improvements in performance of colonoscopy are needed, with particular attention to accurate detection and complete resection of precursor lesions to maximise the protection against CRC.
Introduction
Colorectal cancer (CRC) is a public concern, with 440 000 incident cases and 210 000 deaths in Europe each year.1 ,2 Colonoscopy, with detection and removal of precursor lesions, substantially reduces both the incidence3 ,4 of and mortality5 by CRC, but its protective effect against proximal CRC lags behind.6 A number of studies from Canada and the USA found incidence rates of postcolonoscopy CRC (PCCRC) ranging from 3.4% to 9.0% of all diagnosed CRCs, with a predominant proximal location.7––10
The majority of studies on PCCRCs relied on claims-based administrative data,8––10 thus providing limited information about the contribution of procedural factors (ie, completeness of colonoscopy, potentially missed or incompletely resected lesions). The macroscopic features of PCCRCs, and especially the potential role of flat precursors in the development of PCCRs, have been less studied.11 In particular, non-polypoid (flat or depressed) adenomas can be more easily overlooked in routine practice,12 are more challenging to resect13 and a subset of them have the potential to progress more rapidly to cancer.14 A study by Farrar et al15 conducted in a veteran population showed that PCCRCs are smaller in size and more often proximally located than prevalent CRCs, albeit the macroscopic appearance and aetiology of these cancers was not addressed in their study.
Understanding of the aetiology of PCCRC diagnosed in routine practice, especially the contribution of procedural factors, is of utmost importance as these factors are amenable to correction through educational programmes. In a population-based multicentre study conducted in South-Limburg, we examined the incidence, clinicopathological characteristics and aetiology of PCCRCs diagnosed over a 10-year period. Special attention was paid to the procedural factors (ie, missed or incompletely resected lesions) as these are potentially avoidable.
Methods
Study population and design
We identified all consecutive patients who had been diagnosed with CRC in South-Limburg, the Netherlands from 1 January 2001 to 31 December 2010. Patients with hereditary CRC (ie, Lynch syndrome or polyposis syndromes), inflammatory bowel disease or a previous history of CRC were excluded. As we particularly examined incidence rates and the aetiology of PCCRCs in South-Limburg, we refrained from including external referrals.
Data were collected at three large-volume hospitals (one university and two non-university: Maastricht UMC, Atrium MC Heerlen and Orbis MC Sittard) in South-Limburg. South-Limburg is located in the southeast of the Netherlands, between Germany and Belgium, and has a narrow northern border with the rest of the Netherlands. The region has a total population of approximately 650 000 inhabitants and a low net migration rate of 0.8 per 1000 inhabitants per year.16
For the purpose of this study, we first retrieved all cases diagnosed with CRC using a nationwide digital pathology database (PALGA). We then reviewed digital clinical and histopathology records, including photographic documentation of the CRC resection specimens. We verified the validity and completeness of data using the Netherlands Cancer Registry. A high concordance exists between the pathology database and the Netherlands Cancer Registry.17 ,18
Definitions
We defined PCCRCs as CRCs which had been diagnosed within 5 years after an index colonoscopy; the remaining CRCs were classified as prevalent CRCs. Other authors have used a 3-year interval to define PCCRCs8––10 ,19 ,20; however, in our study we preferred to extend this interval to 5 years as the ‘mean sojourn time’ (ie, the estimated interval between the preclinical (screen) phase and the detectable period21 ,22) may vary with the tumour biology (ie, growth rate) and to achieve the greatest confidence in capturing all PCCRCs.
To assign the most probable aetiology to the identified PCCRCs, we built on an algorithm developed by Pabby et al23and modified by Huang et al.24 We assigned each case of PCCRC to procedural factors (inadequate examination or surveillance, incomplete resection or missed lesions) or tumour biology (newly developed cancers). Inadequate examination was defined as incomplete colonic intubation or poor bowel preparation. Inappropriate surveillance was defined according to the Dutch post-polypectomy surveillance guidelines.25 Incomplete resection was defined as cancer diagnosed in the same anatomical segment as a previously resected advanced adenoma (eg, ≥1 cm in size or containing high-grade dysplasia or a villous component). Missed lesions were considered the main aetiological factor when PCCRCs of any size or stage were diagnosed within <36 months of the index colonoscopy or, in the case of advanced CRCs (size ≥2 cm and TNM stage III/IV), diagnosed in ≥36 months; no previous advanced adenoma had to be found in the same segment at the index colonoscopy. Newly developed cancers were CRCs detected ≥36 months after the index colonoscopy with none or one feature of advanced cancer (large size or advanced stage) and without a previous advanced adenoma in the same segment. Assignment to aetiology was performed by two of the study investigators and, in cases of disagreement, they were discussed until consensus was reached.
The colonoscopic procedure was considered complete when the endoscopist visualised and documented the caecal landmarks. Quality of bowel preparation was classified depending on the endoscopist estimation as sufficient (good or fair) or insufficient (poor).26 ,27 CRCs were categorised according to location into proximal or distal from the splenic flexure and according to their macroscopic appearance into protruded (sessile or pedunculated) or flat.28 ,29 A tumour was considered flat when both the endoscopist and pathologist independently described it as having a non-exophytic, flat or depressed macroscopic appearance. In case of disagreement, the pathologist’s estimation was considered superior. The size of CRCs was routinely measured and documented in the pathology reports. The specialty of endoscopist was subdivided into gastroenterologist and non-gastroenterologist (including gastrointestinal surgeon, general internist or nurse endoscopist).
Study endpoints and statistical analyses
The primary outcome measure was the aetiology of the PCCRCs and secondary outcome measures were the clinicopathological characteristics (ie, location, size, macroscopic appearance and histopathology). Subanalyses were performed according to setting (university vs non-university, gastroenterologist vs non-gastroenterologist) as well as the relation between tumour shape and stage at diagnosis.
Multiple logistic regression analyses using age, gender, location, size, macroscopic appearance, mucinous histology, endoscopist specialty and hospital setting were used to identify potential risk factors for the occurrence of PCCRCs, with a minimum of 10 outcome events (ie, PCCRC cases) per predictor variable as a prerequisite.30 To adjust for possible clustering within the same endoscopist, taking into consideration the variations in number of patients diagnosed with CRC per endoscopist, we used generalized estimating equations (GEE).31 Differences in dichotomous variables were tested using the χ2 test or Fisher exact test, where appropriate. Differences in numerical variables were examined by the independent-samples t test. All ORs were presented with 95% CI. p Values ≤0.05 were considered statistically significant. Data were analysed using the SPSS program V.20.
Results
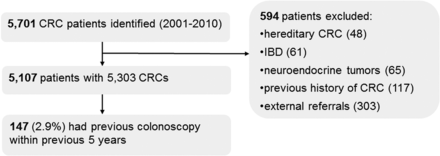
We identified a total of 5701 patients who had been diagnosed with CRC in South-Limburg from January 2001 to December 2010. Figure 1 shows the flowchart of the study. Of the 5107 patients with 5303 CRCs finally analysed, 147 had undergone an index colonoscopy within 5 years before the diagnosis and were considered PCCRCs, accounting for 2.9% of all diagnosed CRCs. The mean (SD) time between the index colonoscopy and diagnosis of CRCs was 26.1 (16.3) months. Table 1 shows the clinical characteristics of patients with PCCRCs and prevalent CRCs. Patients with PCCRCs were significantly older and more often had diverticular disease, coronary artery disease and a family history of CRC than those with prevalent CRCs.
Basic characteristics of patients with postcolonoscopy colorectal cancers (PCCRCs) at the time of diagnosis versus those with prevalent CRCs
Study flowchart, CRC, colorectal cancer; IBD, inflammatory bowel disease.
Index and diagnostic colonoscopy in patients with PCCRCs
The indications for the index colonoscopy were symptoms (ie, anaemia or rectal blood loss) in 74.1%, post-polypectomy surveillance in 22.4% and screening in 3.4% of cases. Of the 147 patients with PCCRCs, 57 had at least one adenoma (mean 1.8, range 1–5), with 33 having at least one advanced adenoma and 90 with no abnormalities at the index colonoscopy.
Overall, 129 patients with PCCRC (87.8%) were diagnosed by colonoscopy while 12.2% were diagnosed during surgery for acute bowel obstruction. Of the 129 patients diagnosed endoscopically with PCCRCs, 73.6% were symptomatic and 26.4% were asymptomatic at the time of diagnosis.
Clinicopathological characteristics of PCCRCs and prevalent CRCs
As shown in table 2, PCCRCs were significantly more frequently located in the proximal colon, were smaller in size and more often had a flat macroscopic appearance than prevalent CRCs.
Clinicopathological characteristics and TNM stage of postcolonoscopy colorectal cancers (PCCRCs) versus prevalent CRCs
Multiple logistic regression analysis, adjusting for age and gender, showed that proximal location (OR 3.92, 95% CI 2.71 to 5.69), a smaller size (OR 0.78, 95% CI 0.70 to 0.87) and flat appearance (OR 1.70, 95% CI 1.18 to 2.43) were independent risk factors for PCCRCs (table 3). As the macroscopic shape of the tumour may be rigorously classified (Paris classification) in early (T1) cancers only, we conducted a sensitivity analysis which showed that early (T1) PCCRCs are indeed more often flat than early (T1) prevalent CRCs (eg, 30.8% (8/26) vs 14.0% (68/486), p=0.040, age-adjusted OR 2.78, 95% CI 1.16 to 6.68). GEE, adjusting for clustering within patients in case of synchronous CRCs, showed similar results (data not shown). We found no significant differences between PCCRCs and prevalent cancers with regard to the presence of mucinous histology, degree of differentiation or TNM stage at diagnosis.
Multiple logistic regression analysis adjusting for age and gender to examine risk factors for postcolonoscopy colorectal cancers (PCCRC)
Aetiology of PCCRCs
In figure 2 the aetiology of PCCRCs is described. Of the 147 cases of PCCRCs, 29 (19.7%) were ascribed to inadequate examination (ie, poor bowel preparation, n=8; incomplete colonoscopy, n=14) or non-compliance with recommended postpolypectomy surveillance intervals (n=7). Of the remaining 118 cases, 13 (8.8%) were attributed to an incomplete resection of an advanced adenoma and 85 cases (57.8%) were attributed to missed lesions. Twenty cases (13.6%) were attributed to newly developed cancers. In table 4 the aetiology of PCCRCs is detailed in relation to the clinical characteristics. Of the 85 PCCRCs ascribed to missed lesions, 52 (63%) were proximally located, 29 (57%) of which were flat.
Aetiology of postcolonoscopy colorectal cancers (PCCRCs) in relation to location and macroscopic appearance
Aetiology of postcolonoscopy colorectal cancers in a South-Limburg cohort.
Rates of PCCRCs in university versus non-university hospitals and relation to endoscopist specialty
Overall, incidence rates of PCCRCs did not differ significantly between the three hospitals (3.1% in the university hospital vs 2.6% and 3.0%, respectively, in the non-university hospitals, p=0.67). The proportions of inadequate procedure/surveillance, missed lesions and newly developed cancers were similar across the three hospitals. However, incomplete resection of an advanced adenoma explained some PCCRCs in the non-university hospitals but none in the university hospital (12.0% vs 0%, p=0.02, Fisher exact test).
Index colonoscopies were performed by 30 gastroenterologists and 9 non-gastroenterologists. The participating non-gastroenterologists were either gastrointestinal surgeons (n=6), general internists (n=2) or specialised nurse endoscopists (n=1). We found no significant association between specialty of practicing endoscopists (ie, gastroenterologist vs non-gastroenterologist) and the occurrence of PCCRCs using a multiple logistic regression model adjusting for age and gender (OR 1.33, p=0.27). GEE were used to examine a possible clustering of PCCRCs within the same endoscopist and taking into consideration the variations in the number of CRC patients each endoscopist contributed to the study; again, no associations were found.
Time trends in diagnosis of CRC and PCCRC
As shown in figure 3, the total numbers of colonoscopies gradually increased over the study period, with a slight increase in the number of diagnosed CRCs. Nonetheless, the number of diagnosed PCCRCs per 1000 colonoscopies remained stable with an average rate of 1.8 PCCRCs/1000 colonoscopies per year.
Time trends in diagnosis of postcolonoscopy colorectal cancers (PCCRCs) in a South-Limburg cohort.
Discussion
In this study we found that the majority of PCCRCs (86%) would most probably have been preventable, being caused by missed or incompletely removed lesions and inadequate examination or surveillance. Of note, we found that PCCRCs were more likely to be proximally located, smaller in size and to have a flat macroscopic appearance than prevalent CRCs, suggesting these could have originated from overlooked precursors at the index colonoscopy. Taken together, these findings strengthen the importance of developing practical skills for accurate detection and resection of all precursor lesions, with special attention to small, flat and proximally located lesions.
We found that procedural factors accounted for the majority of PCCRCs. A twofold failure explained this finding—namely, missed and incompletely removed lesions. With regard to the former, a number of studies now indicate that non-polypoid (flat or depressed) colorectal adenomas contribute to the development of PCCRCs due to overlooked lesions,6 ,32 ,33 a more challenging resection34 or perhaps a more aggressive biological behaviour.13 ,35 Information on clinicopathological features, especially the macroscopic appearance of PCCRCs, is scarce as most studies have relied on registry-based administrative data6 ,8––10 and only a few have been based on clinical data.4 ,15 ,36 Our study is one of the few to examine the clinical features and potential explanations of PCCRCs and is, to our knowledge, the first non-Japanese study to report that a substantial proportion of PCCRCs (31% of the early (T1) PCCRCs and 45% of all diagnosed PCCRCs) had a flat macroscopic appearance.
In line with previous studies, we found that PCCRCs are significantly smaller and more often proximally located than prevalent CRCs.5 ,8 ,9 ,15 As these cancers were diagnosed relatively early after the index colonoscopy (mean interval of 26 months), it is possible that they originated from flat precursors. Early Japanese studies found a predominant proximal localisation of the relatively uncommon but highly malignant depressed lesions,37––39 suggesting these could partly explain the occurrence of PCCRCs.40 In a prospective study at our institution involving endoscopists trained in the recognition of flat lesions,41 ,42 we found that proximally located colorectal neoplasms are more often small and flat than distal ones, thereby contributing to the limited effectiveness of colonoscopy in the proximal colon.
An additional finding of our study is that incomplete polypectomy accounted for 8.8% of all PCCRCs. We specifically focused on the resection of advanced adenomas as up to 35% of these lesions may progress to cancer within 10 years.43 ,44 In a study of 417 polyps resected by experienced gastroenterologists, Pohl et al34 found a comparable rate of incompletely resected adenomas (10.1%, 95% CI 6.9% to 13.3%). Data on the potential impact of incomplete polypectomy on the occurrence of PCCRCs vary widely, ranging from 2.4%27 to 26%.45
In the present study we did not find a significant association between the occurrence of PCCRCs and the specialty of endoscopists or individual clustering of PCCRC cases. This is in line with some studies,15 but contradicts several others which have shown that patients with PCCRCs are more likely to have undergone a colonoscopy by a non-gastroenterologist (ie, a family physician,8 ,9 internist,8 general surgeon10 ,46 or in a non-hospital-based setting8). It is possible that the relative homogeneity with regard to equipment, facilities used and supportive personnel might explain such findings. Notably, in our study, missed lesions accounted for most of the PCCRCs in both university and non-university settings, indicating opportunities for future improvements. In contrast, incomplete resection appeared to be more likely to be a cause of PCCRCs in a non-university than in a university setting.
The incidence rate of PCCRCs in our study was 2.9% of all diagnosed CRCs, corresponding to 1.8 per 1000 colonoscopies. This rate is relatively low and consistent with previous data from the Netherlands,36 thus conferring generalisability for our routine practice. It is, however, difficult to compare the outcomes of different studies with regard to incidences of PCCRCs due to large variations in methodology (definition of PCCRCs, retrospective vs prospective design, differences in populations examined).
In line with previous data,8 ,9 we found that patients with PCCRCs were older and had substantial comorbidity such as cardiovascular disease or diverticular disease. It is plausible that insufficient bowel preparation, which is more common in older and fragile patients with comorbidity, increases the risk of missing lesions.47 ,48 In addition, colonoscopic examination of patients with diverticular disease, some of whom also harbour multiple adenomas,49 is more difficult and colonoscopy might be less effective in preventing cancer. Of note, patients with PCCRC in our study were more likely to have a family history of CRC than those with prevalent CRCs (5.4% vs 1.6%). Although this observation is based on a small number of cases, it emphasises the importance of thorough family history-taking and strict adherence to surveillance guidelines in higher risk groups.
The strengths of our study reside in the population-based design and the use of clinical records and national databases, as well as the use of predefined criteria to retrace the potential aetiological factors of PCCRCs. Our study has several limitations that need to be acknowledged. First, it was retrospective in design and hence the results and conclusions are based on the assumption of reliable data registration across the study period. We attempted to enhance reliability through meticulous documentation and by using validated national registries to reconstruct, as much as possible, the ‘real-life scenario’ underlying the development of PCCRCs. Although we realise that a prospective approach might have been the ideal setting, the relatively low rates of PCCRCs (ie, 1.8/1000 colonoscopies per year in our endoscopy practice) would make it difficult to assemble a large prospective cohort. Second, although some PCCRCs were detected during surveillance, the majority of the patients were diagnosed due to symptoms. We therefore realise we could have underestimated the true incidence of CRCs, as slow-growing cancers which had not yet become clinically overt could have been missed. To minimise this potential bias, we extended the definition of PCCRCs to cancers diagnosed within 5 years after an index colonoscopy. Along with a large sample size, the long-term duration of this study might have mitigated this bias. Third, the precise classification of the shape of CRCs into flat or protruded is difficult, particularly in cases of advanced CRCs as the Paris classification29 is in fact solely applicable to superficial neoplasms. We classified the macroscopic appearance of CRCs in our study based on descriptive data from both endoscopy and pathology records, including photographic documentation. We uniformly applied this definition to all PCCRCs and prevalent CRCs, making it less likely that this factor would have greatly affected the outcome of the study. To mitigate potential bias in appreciation of the tumour shape, we also performed a sensitivity analysis in early-stage (T1) cancers, showing again that PCCRCs were more often flat than prevalent CRCs. Fourth, our study focused on the contribution of procedural factors to the occurrence of PCCRCs and their biological features were not addressed. A few studies reported that PCCRCs are approximately four times more likely to be microsatellite instable and CpG island methylator phenotype (CIMP)-high35 ,50 than prevalent CRCs, suggesting a potential role of the serrated neoplastic pathway. However, none of these studies has been large enough in size or biological scope, and a comprehensive examination of the biology of PCCRCs is therefore awaited. This information may help in the identification of subgroups of patients at higher risk for CRC who may need intensive surveillance.11
In summary, in our experience PCCRCs accounted for 2.9% of all diagnosed CRCs, most of which could be explained by missed or incompletely resected lesions, with a predominant proximal location and a flat macroscopic appearance. Systematic training of endoscopists, with a focus on detection and management of flat precursors, has the potential to prevent PCCRCs.
Acknowledgments
The authors thank the registration teams of the Comprehensive Cancer Center Limburg and the scientific staff of the Netherlands Cancer Registry for assistance with data collection.
References
Footnotes
-
Contributors CMCleC: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript, final approval of the version to be published. MWEB: study concept and design; analysis and interpretation of data; critical revision of the manuscript, final approval of the version to be published. EJAR: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript, final approval of the version to be published. CMB: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript, final approval of the version to be published. ETPK: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript, final approval of the version to be published. RJdeR: study concept and design; analysis and interpretation of data; critical revision of the manuscript, final approval of the version to be published. BW: study concept and design; analysis and interpretation of data; critical revision of the manuscript; drafting of the manuscript, final approval of the version to be published. AAMM: study concept and design; analysis and interpretation of data; critical revision of the manuscript; drafting of the manuscript; obtained funding, final approval of the version to be published. SS: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; study supervision, final approval of the version to be published.
-
Funding CMCleCwas the recipient of an unrestricted educational grant from Pentax BV,The Netherlands. Pentax BVhad no role in the design of the study, data collection, analysis and interpretation, writing of the manuscriptor decision to submit for publication.
-
Competing interests None.
-
Ethics approval The study was approved by the Institutional Review Boards of the participating hospitals and registered in the Netherlands Trial Registry: NTR3093 (http://www.trialregister.nl).
-
Provenance and peer review Not commissioned; externally peer reviewed.