Article Text
Abstract
Objective To characterise the temporal evolution of antibodies to infliximab (ATI).
Design Prospective observational study of infliximab-treated patients with inflammatory bowel disease between 2009 and 2012.
Interventions Trough levels of infliximab and ATI were measured before each infusion by anti-λ ELISA. Patients were monitored for disease activity by clinical activity indexes and for dose-intensification or infliximab cessation. The occurrence of transient ATI disappearing spontaneously without intervention was recorded separately.
Results 125 patients were included (98 Crohn's disease, 27 ulcerative colitis, median follow-up 11.5±22 months) and 1119 sera were analysed for infliximab and ATI levels. Kaplan-Meier analysis showed that 42% of patients remained ATI-free by 4 years of treatment. Most (90%) of the patients who developed ATI did so within the first 12 months of therapy, whereas transient ATI were detected throughout the duration of infliximab therapy (p<0.001). ATI incidence was similar between patients who received infliximab previously (episodic/interrupted therapy patients, n=14) and scheduled-therapy patients (n=111). In the scheduled group, combination immunomodulator+infliximab resulted in longer ATI-free survival compared with monotherapy (p=0.003, logrank test). Survival free of clinical loss of response was enjoyed by 51% of patients, and serial measurements showed that ATI development often preceded the onset of clinical flare.
Conclusions When followed prospectively, most patients who develop ATI do so within the first 12 months of therapy. This incidence is reduced by concomitant immunomodulator even in scheduled-therapy patients. In contrast, transient ATI, which are of little clinical significance, can appear haphazardly at any time during treatment. The onset of clinical loss of response may lag behind the appearance of anti-infliximab antibodies.
- Ibd Clinical
- Infliximab
- Immunology
Statistics from Altmetric.com
Significance of this study
What is already known about this subject?
-
Point prevalence of antibodies to infliximab (ATI) is approximately 60% in episodic infliximab treatment and ranges between 6% and 25% in scheduled treatment.
-
The formation of ATI is associated with lower serum infliximab levels, infusion reactions and in most studies with loss of response.
-
There is scant data regarding temporal evolution of ATI and its correlation to clinical response.
What are the new findings?
-
Most patients who develop ATI do so within the first 12 months of therapy.
-
Transient ATI, which are of little clinical significance, can appear haphazardly at any time during treatment.
-
The temporal evolution of ATI correlates with and often precedes the appearance of clinical loss of response.
-
The incidence of ATI in scheduled-treated patients may be higher than previously appreciated and reach 50% at 1 year. Concomitant immunomodulator prolongs ATI-free survival even in scheduled-treated patients.
How might it impact on clinical practice in the foreseeable future?
-
Immunogenicity risk is low beyond the first year of infliximab therapy and expectant management may be considered when positive ATI are detected after this time point.
-
Pharmacokinetic abnormalities may herald loss of response, providing a rational for investigating pre-emptive interventions to abort soon-to-follow clinical deterioration.
-
Concomitant immunomodulator strategy should be strongly considered for scheduled-treatment patients in view of its potential to reduce immunogenicity.
Introduction
Infliximab (IFX) is a chimeric mouse-human monoclonal immunoglobulin G1 (IgG1) antibody against tumour necrosis factor α (TNFα), which is effective in induction and maintenance of remission in Crohn’s disease (CD) and ulcerative colitis (UC).1–,3 Episodic administration of infliximab elicits the development of antibodies to infliximab (ATI) in approximately 60% of patients.4 In contrast, in patients treated with scheduled infliximab, reported rates of ATI range between 6% and 25%.5 ,6 The formation of ATI is associated with lower serum infliximab levels, infusion reactions and in most studies, albeit not all, also with loss of response.7–,10 Indeed, several aspects of ATI effect on response to infliximab remain obscure as some patients with ATI still benefit from infliximab therapy, while in others loss of response occurs despite the absence of ATI.10–,12
Several explanations have been proposed to account for the differing rates of ATI formation among scheduled-therapy patients and their inconsistent correlation with clinical efficacy, including different assay techniques, lack of uniform definitions and the phenomenon of transient antidrug antibodies.1,1–14 However, another possible cause for these discrepant observations is the diverse and non-comparable time points of ATI measurements along the course of treatment in different studies, which mostly reported point-prevalence of this phenomenon. Indeed, there is a lack of data regarding the temporal evolution of ATI and their incidence during consecutive scheduled infusions of infliximab.9 This knowledge gap severely hampers our ability to interpret the impact of immunogenicity on infliximab clinical efficacy. Therefore, the aim of the present study was to prospectively characterise the temporal evolution of antidrug antibodies in patients with inflammatory bowel disease (IBD) treated with infliximab and to explore its correlates with the onset of clinical loss of response.
Methods
Patient population and outcome definitions
This was a prospective observational study of patients with IBD attending the Gastroenterology Department of the Sheba Medical Center and receiving infliximab infusions between February 2009 and February 2013. Pre-infusion sera were obtained in all patients before each infusion and analysed for infliximab trough level and for ATI level prospectively. Infliximab and ATI levels were considered positive if they were above the assay's detection threshold: >0.6 µg/mL, >2.5 µg/mL-equivalent (-eq) respectively.
Patients in whom sequential sera were unavailable because of receiving infusions at out-of-hospital infusion centres were excluded, as were patients who refused to consent. Patients who started scheduled infliximab infusions before study initiation were also excluded, unless they entered the study period receiving the standard 5 mg/kg/8 weeks regimen without evidence of ATI or undetectable infliximab and without prior dose intensification.
The study was approved by the Sheba Medical Center Ethics committee and all patients gave an informed consent to participate. Clinical response was determined by the HBI (Harvey-Bradshaw index) and by the SCCAI (simple clinical colitis activity index) on the day of infliximab infusion, for patients with CD and UC, respectively. A response to therapy required a drop>3 points in either the HBI or SCCAI and remission was defined as a HBI of ≤4 or SCCAI ≤2.15–17 When unavailable, clinical response was determined by the documented physician global assessment of the patient.
Infliximab trough levels and ATI levels were correlated with several clinical endpoints. These included clinical response, primary non-response, secondary loss of response, drug discontinuation and infusion reactions. Clinical response was defined by an improvement in disease activity indexes as outlined above in a steroid-free patient and coupled with a treating physician decision to continue scheduled therapy of infliximab without alteration. Primary non-response was defined as lack of improvement at the end of the induction coupled with a decision to stop infliximab. Secondary loss of response was defined by re-emergence of disease activity (a rise of >3 points in HBI score or of >2 points in SCCAI score) after achieving an appropriate induction response, coupled with a need for alteration of therapy (dose intensification or addition of an immunomodulator) or a need for infliximab discontinuation.18 Physicians were not kept blinded to ATI results. Severe infusion reactions referred to serious infusion reactions which necessitated cessation of infliximab therapy or addition of an immunomodulator. Antibody formation was defined as positive when a patient tested positive for ATI during follow-up on more than two consecutive time points. Transient antibodies were defined as measurable ATI on up to two consecutive infusions which disappeared on subsequent infusions without any alteration of therapy. These were analysed separately.
Determination of infliximab levels
Hundred µlitres of 1:100 diluted serum was added to preplated 750 ng/mL TNFα (Peprotech, Rocky Hill, New Jersey, USA) and incubated for 90 min. Following washing, horseradish peroxidase (HRP) labelled goat antihuman Fc fragment antibody (MP Biomedicals, Solon, Ohio, USA) at a concentration of 0.62 µg/mL was added for 60 min and reacted with tetramethylbenzidine (TMB) substrate. The results were then read on an ELISA reader. Quantisation of the measured infliximab concentration was done by calibration to standard curve in which exogenous infliximab (Schering Plough, New Jersey, USA) was added at concentrations between 3 ng/mL and 200 ng/mL.
Measurement of ATI
ATI were determined as previously described.7 ,19 Briefly, infliximab (0.1 mg/mL) was added to preplated TNFα (500 ng/mL) in 100 µL wells of ELISA plates (Nunc, Roskilde, Denmark). After drying, 100 µL of serum was added and incubated for 90 min at room temperature. Plates were then washed and goat antihuman λ chain HRP-labelled antibody (Sertec, Oxford, UK) was added at a dilution of 2.5×104 for 60 min and reacted with TMB substrate. The results were read by an ELISA reader EL-800 (Biotek Instruments, Winooski, USA) and expressed as mcg/mL-eq after normalisation versus results obtained using additions of graded concentrations between 9 ng/mL and 600 ng/mL of HRP-labelled goat antihuman F(ab′)2 fragment antibody (MP Biomedicals).
Statistical analysis
Continuous variables were analysed by a two-tailed Student t test or the Mann-Whitney U test, as appropriate. Correlations were analysed by the Spearman's rank correlation test. Categorical variables were analysed by Fisher's exact test. OR and 95% CI were computed for all variables compared. Kaplan-Meier curves were plotted to assess the temporal rate of events and logrank test was computed for the comparison between survival-free durations. All statistics were performed using MedCalc software (V.12.2.1.0, Mariakerke, Belgium). A two-tailed p<0.05 was considered statistically significant.
Results
Patient population
Out of 182 patients who received infliximab during the study period, 42 patients were excluded due to missing serum samples (19 had started infliximab before the study was initiated and 23 received infusions outside our centre), 10 were lost to follow-up and 5 patients refused to consent. Thus, 125 patients were included (98 CD, 27 UC, median follow-up time 11.5, IQR=3.5–22 months) and 1119 sera samples were analysed for infliximab and ATI levels during the 4-year study. The patients’ background disposition and clinical characteristics are presented in table 1.
Background disposition and clinical characteristics
Incidence of ATI formation over time
Fifty-eight out of 125 patients (46%) developed ATI. The first detection of ATI occurred at a mean of 4.5 months after therapy initiation. The median time was 1.5 months (IQR=0.5–5.5, range 0.5–31 months), implying that 75% of patients developed ATI by week 22. Positive ATI of any value were significantly predictive of concomitant undetectable drug level (OR 2.9, 95% CI 2.1 to 3.9, p<0.0001, see online supplementary figure S1). This correlation with nil drug levels was more robust for high ATI above 8 mcg/mL-eq (OR 29.5 95% CI 7.2 to 120, p<0.0001).
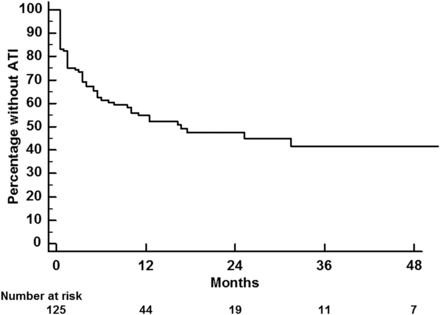
Kaplan-Meier analysis showed that ATI-free survival was experienced by 42% of patients by 4 years of follow-up (figure 1). Interestingly, 52/58 (90%) of the patients who developed ATI did so within the first 12 months of therapy.
Survival free of the development of ATI in the whole cohort. ATI, antibodies to infliximab.
The incidence of ATI formation was similar between patients previously exposed to infliximab (episodic/interrupted therapy patients) and scheduled-therapy patients (n=14 and n=111, respectively, p=0.6, logrank test, figure 2A). In the scheduled-therapy group, combination of infliximab with an immunomodulator resulted in significantly longer ATI-free survival compared with monotherapy (p=0.003, logrank test, figure 2B). An additional analysis showed a trend for lower median ATI titres on most time points up to week 54 in patients treated by combination versus monotherapy, but the difference was blunted thereafter. A similar trend was observed for higher infliximab levels up to week 38 (data not shown).
(A) Survival free of ATI development in patients with episodic/interrupted treatment versus those receiving scheduled infliximab infusions. (B) Survival free of ATI development in scheduled-treated patients receiving infliximab monotherapy versus those receiving combination infliximab+immunomodulator. ATI, antibodies to infliximab.
Interestingly, analysis of the 98 patients with CD versus the 27 patients with UC showed that ATI-free survival was longer in patients with CD (p=0.02, logrank test). However, on multivariate analysis also incorporating concomitant immunomodulator and episodic/interrupted therapy as parameters in the model, the difference between CD and UC did not retain its independent significance (data not shown).
In contrast with our λ-chain detection ELISA, most commercially available ATI ELISA assays employ infliximab as a detection antibody and are unable to measure ATI in the presence of infliximab.19 In agreement with a previous publication19 we found that the incidence of loss of response, and the temporal evolution of ATI were not different between patients with an ATI positive infliximab negative (ATI+/IFX-) status (51 patients in total) compared with all 58 ATI positive patients, including the 7 patients who demonstrated the ‘double positive’ (ATI+/IFX+) status.
Clinical response and its temporal evolution
Fourteen out of 125 (11%) of patients had primary non-response to induction and stopped infliximab. Survival free of clinical loss of response was enjoyed by 51% of patients by the end of the study period. Fifty out of 125 (40%) of patients were followed for at least 1 year (54 weeks) without clinical loss of response (median 98, IQR=72–164, range 54–624 weeks). Low infliximab trough levels and high ATI titres were significantly more prevalent among patients with loss of response (figure 3A,B respectively, p<0.001). Kaplan-Meier survival curve was performed for patients with high ATI versus patients with low and absent ATI (figure 3C). As could be expected, survival free of loss of response was significantly longer among patients with no ATI (logrank test, p<0.01). However, there was no statistically significant difference between patients with high versus low ATI.
(A) Infliximab trough level measurments at time points of response or loss of response. Each dot represents an individual time point of infliximab trough level measurement. (B) ATI titre measurments at time points of response or loss of response to infliximab. Each dot represents an individual time point of ATI level measurement. (C) Survival free of clinical loss of response to infliximab in patients with high titre versus low titre versus absent ATI. ATI, antibodies to infliximab.
Because episodic therapy patients are known to benefit from combination therapy, we decided to investigate if combination therapy was also clinically superior in patients receiving scheduled therapy. This analysis, performed after excluding the episodic/interrupted therapy patients, still showed a statistically significant longer duration of clinical response to infliximab in patients receiving combination compared with monotherapy (p<0.01, see online supplementary figure S2A). This benefit was blunted to a borderline significance level (p=0.06) when only scheduled patients in the maintenance therapy phase were considered (see online supplementary figure S2B).
Chronological correlation between ATI and clinical loss of response
During the prospective follow-up of this cohort it was noticed that some patients seem to develop absent infliximab and rising ATI levels without loss of response. This prompted us to investigate the chronological sequence of events of ATI and clinical loss of response. This analysis showed that in ATI+ patients, there was a significant correlation between timing of ATI formation and loss of response, but the power of the correlation was not robust (r=0.62, 95% CI 0.41 to 0.77, p<0.001, figure 4A). To elucidate, at least partly, the reasons for the suboptimal temporal correlation between ATI formation and clinical loss of response we plotted the time interval between first detection of ATI as the reference point at time 0, and the relative timing of loss of response in patients who developed ATI and loss of response (figure 4B). This showed that in many patients (54%), the detection of ATI preceded the onset of clinical loss of response (median delay of 2 months, median duration from infliximab initiation to loss of response—3.5 months). In 30% the two events were detected simultaneously, and in 16% of the patients an opposite phenomenon was observed, whereby ATI lagged after first loss of response (median delay of 2.5 months, median duration of 11 months from infliximab initiation). Interestingly, all four patients who lost response and were dose-intensified prior to ATI appearance subsequently developed low-titre ATI levels. In one of them this was accompanied by undetectable drug levels and three had measurable drug levels. None of the patients developed high ATI on consecutive samples.
(A) Scatter plot depicting time points of ATI detection and the onset of LOR in scheduled-therapy patients. Each circle denotes an individual patient. (B) Time intervals between the onset of measurable ATI (normalised to be set at time 0) and the onset of clinical LOR for all individual patients in the maintenance treatment group who developed ATI and LOR. ATI, antibodies to infliximab; LOR, loss of response.
Transient ATI
Of the study cohort 26% developed transient ATI which disappeared within two consecutive infusions and were not associated with a need for a change in therapy. In 52% of those patients transient ATI recurred on non-consecutive measurements. The temporal distribution of ATI in comparison with transient ATI is shown in figure 5. As mentioned, most (90%) of the patients who developed non-transient ATI did so within the first 12 months of therapy, whereas transient ATI were detected throughout the duration of infliximab therapy. The median time to development of transient and non-transient ATI was 13.5, IQR=7.5–33 months and 1.5, IQR=0.5–5.5 months, respectively (p<0.001).
Time points of antidrug antibodies detection events for non-transient ATI compared with transient ATI, expressed in months following infliximab initiation. Each triangle denotes an individual patient. ATI, antibodies to infliximab.
Discussion
This study aimed to prospectively investigate the temporal evolution of antidrug antibodies (ATI) in patients with IBD treated with infliximab and to explore the correlation between ATI formation and the onset of clinical loss of response. Despite ample research on the point prevalence of ATI, their incidence and temporal evolution during therapy have not been previously well defined. It is known that episodic administration of infliximab elicits induction of ATI in approximately 60% of patients.4 Among patients with IBD receiving scheduled infliximab therapy a wide range of ATI formation rates, between 6% and 25%, has been reported.5 ,6 ,20 This wide range of ATI rates among the scheduled-therapy studies has been attributed to diverse patient populations and different assays employed.12 ,14 However, the increase in the rate of ATI formation over time observed in the present study suggests that the discrepant ATI frequencies between different scheduled-therapy studies may also partly stem from study designs which reported ATI point prevalence rates measured at different time points of infliximab therapy. In addition, the findings of the present study and those of another study using a homogenous mobility shift assay (HMSA)13 suggest that the true incidence of immunogenicity among scheduled-therapy patients may be higher than previously appreciated and approximate 50% by 1 year of therapy.
An important additional observation is that 90% of the patients who developed ATI did so within the first 12 months of therapy. Baert and colleagues have demonstrated that ATI incidence did not seem to increase on further infusions after the fifth infusion, although their cohort was mostly composed of episodically treated patients and Kaplan-Meier analysis was not formally performed.4 In ACCENT1 the cumulative incidence of ATI in the scheduled-therapy group seemed to peak at week 54, although not all time points were sampled, no samples were available beyond week 72 and only 9% of the maintenance therapy group developed ATI suggesting a technical limitation of the double antigen ELISA assay.5 Nonetheless, taken together, these observations indicate that scheduled-treated patients are at low risk of infliximab immunogenicity if 12 months of ATI-free therapy have elapsed. This observation may also explain the puzzlingly low rate of immunogenicity-driven adverse effects in patients who received retreatment in the STORI trial21 because successful completion of 12 months of infliximab therapy probably identifies a selected subgroup which has a low risk for developing immune sensitisation to the drug later on and is hence protected from a non-response (or from an infusion reaction) to retreatment. The fact that immune sensitisation to infliximab mostly occurs in the first 12 months may also partly explain the lack of superiority of combination therapy beyond 6 months which was reported in the IMID trial, although several other factors may play a role in that particular study's findings.22
It is of note, that no difference in ATI-free survival was demonstrated between scheduled and episodic/interrupted therapy in our cohort. This probably arises from the small number of episodically treated patients, or from the fact that unlike ‘canonical’ episodic patients, most of our episodic patients were in fact interrupted therapy patients who had previously received infliximab for prolonged periods and discontinued it while in remission (eg, during pregnancy). The high rate of ATI among scheduled-therapy patients may have further blunted this difference.
The effect of immunomodulators on ATI formation is controversial. While most recent studies detected significantly lower incidence of ATI among patients who were concomitantly treated with immunomodulators, few reported that concurrent immunomodulators have no effect on the rate of ATI formation.4 ,6 ,23 ,24 In a previous study we have demonstrated that combination therapy might even eliminate existing ATI and restore clinical response.25 In the present study combination therapy resulted in significantly longer ATI-free survival compared with monotherapy. Furthermore, similarly to the findings of the SONIC trial24 combination therapy also significantly prolonged survival free of loss of response even in the scheduled-therapy patients. Interestingly, this effect was more pronounced during early treatment weeks suggesting a beneficial role of combination therapy already during induction, which was also indicated by the SONIC data. Moreover, an analysis of the temporal evolution of infliximab and ATI median levels showed a trend for difference between combination and monotherapy only until week 38 and week 54, respectively, further indicating the rare occurrence of new-onset immunogenic pharmacokinetic derangements beyond this time point.
The effect of ATI formation itself on clinical loss of response is still debated. Many have shown a correlation between ATI formation and loss of response, while some investigators could not corroborate the existence of such correlation.4–6 8–11 ,20 In our study, positive ATI and low infliximab trough levels were significantly more prevalent among patients with clinical loss of response. However, the rate of loss of response was similar for patients with low or high ATI (>8 µg/mL-eq). This might seem peculiar, as high ATI titres are considered more strongly correlated with infusion reactions and worse clinical outcome, and could stem from technical variations between the different assays used to measure ATI titres.4 ,26 Nevertheless, low titre ATI, especially when accompanied by low drug level, has also been linked to clinical loss of response.18 Once transient antibodies were excluded, most of our ATI+ results, whether low or high titre, were accompanied by undetectable drug level. It is possible, that low drug levels lead to a similar rate of loss of response regardless of the ATI titres.27 This is not the same as the response to dose escalation which may be better for low-titre ATI patients compared with high-titre ones,28 but more data is required to resolve this issue.
While there was a significant temporal correlation between ATI formation and loss of response, in 54% of the patients ATI formation preceded clinical loss of response by a median of 2 months (figure 4B). It is well known that several weeks of anti-TNF therapy are required at induction before inflammation subsides and clinical response becomes evident.1 ,29 The above findings suggest that in some patients, a similar time lag may occur between the first appearance of immunogenicity and the re-emergence of tissue inflammation and ensuing clinical symptoms. These results also indicate that at least some of the cases reported wherein ATI are present in the absence of loss of response, may in fact be patients in whom resurgence of inflammation has not yet reached the threshold burden at which clinical relapse becomes apparent. Notwithstanding, more large-scale studies are needed in order to determine whether consistent low infliximab levels or the appearance of ATI should alert the physician to consider dose optimisation even prior to onset of clinical loss of response. Indeed, a clinical trial exploring the efficacy of pre-emptive pharmacokinetic-driven dose optimisation approach is now underway in Belgium.30
Transient ATI were detected throughout the duration of infliximab therapy, in contrast with non-transient ATI, which appeared mostly during the 1st year of follow-up. Two recent studies have found that patients with transient ATI are less likely to discontinue infliximab for loss of response or to experience hypersensitivity reactions compared with patients with sustained ATI.13 ,31 The present results add to this concept, by showing that a positive ATI result observed beyond 12 months of therapy is more often than not a transient ATI phenomenon of little clinical consequence. Thus, in practical terms, the majority of positive ATI measurements at late time points do not necessarily reflect a genuine evolving immunogenicity. Whether alteration of therapy is necessary in such patients should be weighed against the option of expectant management with repeated measurements, and be based on the severity of symptoms and clinical judgment.
There are several limitations to our study. First, the results were obtained with the extensively validated anti-λ detection ELISA,7 ,19 ,25 but corroborating studies using other assays are pertinent as measurements may not be entirely comparable. Second, the study population consisted of 125 patients but the follow-up time of patients in this observational study was variable, which should also be borne in mind when interpreting the results. Also, because treating physicians were not kept blinded to the ATI results which were performed routinely during this observational study, one cannot exclude that the assays’ results may have influenced clinical management. However, since our primary outcome was the emergence of ATI, whatever the interventions physicians later implemented based or not on such detection of ATI should not in itself impact the main study outcome.
In conclusion, 90% of the patients who develop sustained ATI do so within the first 12 months of therapy whereas transient ATI can be detected throughout the duration of infliximab therapy. Therefore, immunogenicity risk is relatively low beyond 12 months of infliximab therapy and expectant management may be considered for positive ATI detected after this time point depending on the clinical setting. The temporal evolution of ATI correlates with and may precede in time the appearance of clinical loss of response, and both events are delayed by combination therapy even in scheduled-treated patients. Future studies will need to define whether interventions motivated by pharmacokinetic measurements alone can abort such soon-to-follow clinical loss of response.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online figures
Footnotes
-
Contributors SBH conceived the study and drafted the manuscript. BU interpreted the results and drafted the manuscript. MY, OP, EF, OH-N, UK, YC and RE participated in acquisition of data and critical revision of the manuscript for important intellectual content.
-
Funding The research leading to these results was partly supported by the ‘Talpiot’ Medical Leadership program of the Sheba Medical Center (to SBH), and by the Innovative Medicines Initiative Joint Undertaking under grant agreement number 115303 of the ABIRISK consortium, resources of which are composed of financial contribution from the European Union's Seventh Framework Program (FP7/2007-2013) and in kind contribution of EFPIA companies (to SBH and YC).
-
Competing interests SBH and YC have served as consultants to Abbot and Schering-Plough and have received unrestricted educational grant from Janssen.
-
Ethics approval Sheba Medical Center Ethics committee.
-
Provenance and peer review Not commissioned; externally peer reviewed.