Article Text
Abstract
The cervical inlet patch is an island of heterotopic gastric mucosa, most commonly found in the proximal oesophagus. Its importance as a cause of throat symptoms has been recognised, particularly chronic globus sensation. This has led to a change in the Rome IV criteria for globus management, with emphasis on ruling out the condition. Proton pump inhibitors are often ineffective in resolving symptoms. Endoscopic studies on the use of ablative techniques, most recently radiofrequency ablation (RFA), have shown promise in reversing the CIP to mormal squamous mucosa, with subsequent symtpomatic resolution.The aim of this review is to update on the investigation and management of the CIP.
- oesophageal disorders
- acid-related diseases
Statistics from Altmetric.com
Introduction
The cervical inlet patch (CIP), also known as ‘gastric inlet patch', is an island of heterotopic gastric mucosa located in the postcricoid portion of the oesophagus at or just below the upper oesophageal sphincter. It is a congenital condition first described over 200 years ago by Schmidt.1 Its importance as a cause of throat symptoms has been recognised, and led to a change in the Rome IV criteria for globus management, with emphasis on ruling out the condition.2 Studies have shown ablative techniques to be effective in their management, most recently radiofrequency ablation (RFA).3 The aim of this review is to update on the investigation and management of the CIP.
Prevalence
Studies report variable figures regarding the presence of heterotopic gastric mucosa in the proximal oesophagus. The largest autopsy series (1000 cases) demonstrated a prevalence of 4.5% in children.4 Several studies estimated CIP prevalence at between 0.03% and 5.9% in children.5 Schridde found microscopic foci present in the inlet area in 70% of autopsies.6 The prevalence is similar in different age groups of patients7 and thought to be equal in both sexes8 although a few studies found that CIP is more common in men.9 10
In adults undergoing diagnostic conventional oesophagogastroduodenoscopy, incidence ranges between 0.1% and 10%.7 8 11 12 It is likely that this variability is due to the quality of endoscopy. The identification of an inlet patch has been correlated with the awareness of the endoscopist, with one study demonstrating the detection rate falls from 2.27% to 0.29% when endoscopists studying prevalence were compared with those who were unaware.7 In contrast, in patients with throat symptoms undergoing transnasal endoscopy in an Ear Nose & Throat (ENT) setting, the yield was zero, despite more than 800 patients in three studies.13–15 It is therefore assumed that prevalence of CIP is underestimated.
Pathogenesis
The pathogenesis of CIP is not known. The most accepted hypothesis assumes that CIP has a congenital origin and is a consequence of incomplete transformation from columnar to squamous epithelium during embryonic development.7 At the 11 weeks of embryonic stage, the squamous lining replaces the columnar lining starting from the mid-oesophagus and extends both proximally and distally. The proximal oesophagus is, therefore, the last part to undergo transformation and this explains the presence of heterotopic gastric mucosa at this site.16 17 Supporting evidence comes from immunohistochemical analysis showing that CIP contains glucagon reactive cells that are not found in the mature human stomach, but are seen in its embryonic form.18 Furthermore, the CIP is frequently discovered in children and its prevalence does not increase with age.8 12
A less commonly held theory involves rupture of occluded proximal oesophageal glands that lead to retention cysts which may burst and result in heterotopic gastric mucosa.19 A third theory proposes chronic acid exposure-related metaplastic transformation of squamous to columnar lining.20
Histology
Studies have shown that the most common histological subtype is fundic mucosa (gastric body type with oxyntic glands, acid-secreting parietal and chief cells)4 11 21 followed by cardia type mucosa (figure 1). Gastric parietal cells in some inlet patches are able to secrete an amount of acid.22–25 The patches are in close proximity to the larynx and pharynx, and the acid secretion can reflux into this area and cause symptoms. Hyperacidity can also induce chronic inflammation and ulceration, and oesophagitis commonly coexists in the squamous mucosa surrounding the CIP on histology.21 26 Less frequently this can lead to oesophageal strictures and webs.
Histological types of inlet patch: oxyntic mucosa (left) and cardiac mucosa (right) (Images courtesy of Dr Fuju Chang).
Rarely, histopathological examination of CIP shows a ‘transitional’ cell type with a random mixture of fundic and antral glands or an ‘antral’ pattern, defined by the absence of chief cells and only a few parietal cells.11 Microscopically, inlet patches commonly have an associated inflammatory infiltrate,8 with granulocytes and plasma cells. Other histological changes including atrophy, metaplasia, dysplasia and adenocarcinoma of the CIP are very rare.17 27
Symptoms
Many patients with CIPs are asymptomatic, and diagnosis is made incidentally during upper gastrointestinal endoscopy.7 28 29 Nevertheless, symptoms reported in both the adult and paediatric population are probably related to the acid or mucus produced by the patch and include globus, cough, sore throat, hoarseness and excessive throat clearing. The most commonly associated condition that causes this cluster of symptoms is laryngopharyngeal reflux (LPR).
Table 1 summarises studies that have assessed the prevalence of LPR symptoms between patients with and without CIP. In the largest of these, a retrospective study which included 487 229 endoscopies, Neumann et al 30 showed that dysphagia, odynophagia, upper respiratory symptoms and globus were significantly more common in patients with CIP. Most show an increased presence of LPR symptoms,24 30 31 particularly globus sensation. It is because of this that the Rome IV diagnostic criteria for investigation of globus sensation ‘has been modified relative to the recent insights into the gastric inlet patch in globus symptom generation, and a concerted effort to support endoscopic evaluation of the oropharynx’.2
Prevalence of LPR symptoms in patients with CIP
The size of the patch has been shown to correlate with symptom severity, possibly as a result of increased acid secretion.24 32 Other authors found significantly more chronic cough among patients with larger patches, whereas smaller patches size were associated with more sore throat.31
In children with CIP, symptoms tend to differ from the adult population. There is a higher incidence of respiratory symptoms, specifically cough, wheezing and asthma in some,33 and of dysphagia and food impaction in others.5 It should be noted however that these studies were published before the diagnostic criteria for eosinophilic oesophagitis were established, another important change to the Rome IV diagnostic criteria for globus.
Investigation
Upper GI endoscopy
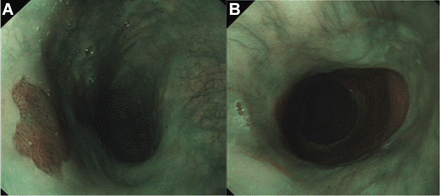
The diagnosis is based on the endoscopic finding of a salmon coloured patch, separate from the Z-line and unrelated to Barrett’s oesophagus. The CIP is usually found at between 15 and 21 cm from the bite guard, and appears as a velvety, rose or salmon coloured ovoid or round patch, with clearly defined borders from the surrounding greyish pearly coloured oesophageal mucosa (figure 2A). The proximal part of the oesophagus is best examined when slowly withdrawing the endoscope, with repeated short inflations while rotating the instrument clockwise or anticlockwise. The patches vary in size from microscopic to as large as 5 cm and can be single or multiple (figure 2B). Circumferential patches have been described (figure 3). They are usually flat but may appear slightly depressed or raised, with a smooth or micronodular surface. Rarely, raised polypoid patches have been described.34 Small lesions may be covered with oesophageal squamous epithelium without discernible changes in the overlying oesophageal mucosa.35
(A) Salmon coloured patch of mucosa found in the proximal oesophagus, just distal to the upper oesophageal sphincter. (B) On occasion, more than one inlet patch may be present.
(A) Small inlet patch in the cervical part of the oesophagus, left wall narrow band imaging (NBI) and (B) circumferential inlet patch (NBI).
The value of routine biopsy of the inlet patch has not been established. In a large study of 173 809 patients who had oesophageal biopsies, Lujan et al revealed that there was a strong association between the endoscopic suspicion of an inlet patch and its histopathological confirmation (positive predictive value 0.53). Whether the histology changes the management of the inlet patch, for example, if predominantly gastric type mucosa then a more concerted attempt to treat with proton pump inhibitor (PPI), has not been studied.28
Role of virtual chromoendoscopy
A number of studies show that endoscopy with narrow band imaging (NBI) (Olympus, Japan) increases the detection rate of inlet patches about threefold compared with standard white light endoscopy.36 NBI (figure 3) improves the detection of small lesions (54% vs 17%, p<0.0001 in a prospective study of 99 patients with CIP).37 It is recommended to confidently rule out an inlet patch using virtual chromoendoscopy in light of this.
Radiological findings
Although not recommended to rule out an inlet patch, a barium swallow can show characteristic features, which are often ordered in the diagnostic work-up of a patient with globus from ENT clinics. The most common pattern consists of small indentations on the wall of the oesophagus. The indentations may be more prominent with an intervening bulge away from the oesophageal lumen. Other possible findings reflect the prominent border of the inlet patch and include rim-like shadows and irregular outlines.38
Acid exposure and motility
Oesophageal pH monitoring in patients with CIP has revealed a wide spectrum of results, including increased distal and proximal oesophageal acid exposure, longer bile exposure time in the distal oesophagus and episodes of acid secretion from the patches which are not related to gastric acid exposure.22–25 Isolated acid exposure has been demonstrated on 24-hour dual-channel pH studies in the pilot study of RFA.3 An early study by Hamilton et al demonstrated isolated acid exposure by using Congo red staining after stimulation with pentagastrin.39
A study on manometry by Rosztóczy et al 22 reported that the lower oesophageal sphincter (LOS)showed significantly decreased pressure, length, abdominal length and longer relaxation time whereas in the oesophageal body the amplitude of peristaltic waves was decreased and the number of simultaneous contractions was increased. Korkut et al 23 also showed that some patients with CIP have signs of oesophageal motor dysfunction based on manometry. These findings suggest that patients with CIP also have motility disorders contributing to their symptoms.
Associations
Inlet patch and Barrett’s oesophagus
The association between heterotopic gastric mucosa and Barrett’s oesophagus has been a subject of debate in many studies which have reported conflicting results.
Four studies26 27 30 40 have reported significant positive associations, while seven other studies have not found any association.8 12 27–29 31 32 Avidan et al found in a large case–control study (53 patients with inlet patch and 4882 control subjects) that Barrett’s oesophagus was four times more common in patients with CIP than control patients without CIP (34% vs 9%).40 This has led to theories regarding a common pathogenesis between CIP and Barrett’s oesophagus, where an incomplete development of the squamous epithelium leaves isolated gastric-type cells or patches at either end of the oesophagus. There is limited evidence to support this view, however, with a few studies showing limited agreement in COX 2 staining patterns between Barrett’s oesophagus and CIP.41 42 At present, the evidence only reports an association rather than a shared pathogenesis.
Adenocarcinoma arising from CIP
Neoplastic progression from a CIP is extremely rare. Only 47 cases of adenocarcinoma associated with CIP have been reported in the literature to date.43 It is estimated that the lifetime incidence of neoplasia among patients with CIP ranges between 0% and 1.56%.20 30 This is low compared with Barrett’s oesophagus which confers a lifetime risk of developing adenocarcinoma of 3% in women and 5% in men.44 The presence of inflammation, atrophy, metaplasia and dysplasia within CIP led some authors to propose a metaplasia-dysplasia-carcinoma sequence, similar to Barrett’s.16 45 A literature review based on 43 cases reveals that a majority of those affected were men (88.4%) with a median age of 60.4 years (range 35 to 85 years), with dysphagia reported as the most common symptom (74.4%) and with little acid-related symptoms.17 One confounder may be tobacco smoking, which in a general population study involving 822 patients, was positively associated with the presence of an inlet patch46
H. pylori and inlet patch
Inlet patches may be colonised by Helicobacter pylori, with a prevalence as high as 82% in those with H. pylori of the stomach.47 A high prevalence in the CIP was directly correlated with a high density of H. pylori in the stomach7 47 As H. pylori is transmitted via the oral route, the proximal location of CIP in the upper gastrointestinal tract may make this site more prone to colonisation. Another proposed mechanism is colonisation from antrum through gastro-oesophageal reflux. Given these findings, performing a test for H pylori in patients with CIP may be considered, although there is a paucity of data to support this approach.
Other associations
Benign complications of CIP reported in the literature are rare but include strictures, web, ulceration, bleeding, fistula with or without subcutaneous abscesses, perforation and polyps.17
Therapeutic approach
Proton pump inhibitors
There are no standardised treatment strategies for CIP. Asymptomatic patients with an incidental finding of CIP do not require any treatment. Progression to malignancy or complication is extremely rare, and patients should be advised with regard to this when the diagnosis is made. Acid suppressive therapy with PPI may improve symptoms but has not been proven in a trial setting.
Endoscopic therapy
Argon plasma coagulation
In a pilot study, Meining et al demonstrated argon plasma coagulation (APC) to be effective for symptom resolution in a patient with globus secondary to CIP, although with a short follow-up of 2 months.48 This was undertaken using a cap to assist stabilisation of the mucosa, with a power setting of 60 W and argon flow set to 2 L/min. A prospective randomised controlled trial from the same group followed and demonstrated that 82% of patients treated with APC had a significant improvement of globus sensation and 90% had complete endoscopic healing versus zero in the sham procedure group.49 Stricture formation, bleeding or perforations did not occur. In a follow-up study after a median period of 27 months, APC was assessed as a successful therapy in 74% of patients.50 In a meta-analysis of six studies on symptomatic CIP treated by APC, a response to therapy was documented in more than 80% of patients. The median follow-up in these studies ranged from 1 to 36 months.49
Endoscopic mucosal resection
Endoscopic mucosal resection has been described in one case of high-grade dysplasia51 and in six patients for superficial oesophageal adenocarcinoma arising from the CIP.45 In two of these patients, there was no evidence of disease at 12 and 31 months follow-up, respectively.
Endoscopic submucosal dissection
Endoscopic submucosal dissection (ESD) was performed by Katoda et al in a patient with a submucosal adenocarcinoma arising in CIP.45 The patient declined further intervention and opted for surveillance. Risks of strictures in this area would be considered very high following ESD.
Radiofrequency ablation
A novel technique using an RFA through the scope (TTS) catheter was evaluated by Dunn et al in a prospective pilot study of 10 patients.3 All had histologically proven CIP with symptoms of chronic globus sensation and/or a sore throat, ENT review with laryngoscopy, high-resolution manometry and 24-hour two-channel pH monitoring. Mean number of CIP was 2 (range, 1–4) with a median surface area of 2 cm2 (range, 0.5–14 cm2). After a median of two RFA sessions, 80% achieved complete endoscopic and histological resolution and the remainder had >90% visual resolution (figure 4). Globus, sore throat and cough were significantly improved from baseline and were durable for a median follow-up of 15 months. There were no complications or buried glands identified at follow-up. RFA is associated with a low risk of strictures (<5%), bleeding (<2%) and perforation (<1:1000).
Cervical inlet patch before and after ablation with RFA from clockwise, (A) inlet patch at 10 o’clock position, (B) immediately after first RFA (TTS), (C) at 3 months after first ablation there is squamous regeneration but a tiny central foci remains. (D) complete squamous regeneration at 3 months postsecond RFA. RFA, radiofrequency ablation.
Conclusion
CIP is an often underestimated congenital condition that is clinically relevant due to its association with throat symptoms, which are commonly referred to gastroenterology clinics. Gastroenterologists should be aware of the association, particularly in patients with chronic globus sensation, and make efforts to carefully inspect the proximal oesophagus using high definition endoscopy with NBI. Although a trial of PPI may be effective, newer endoscopic techniques are emerging to treat the condition, with high rates of efficacy and durability. Despite being described over 200 years ago, many questions regarding the CIP remain unanswered. Research into pathogenesis; associations with Barrett’s oesophagus; prevalence in asymptomatic and symptomatic cohorts; symptom correlation with oesophageal physiology findings; and efficacy of novel endoscopic therapies is warranted.
References
Footnotes
Contributors RR and JMD drafted manuscript; SI and TW edited and revised manuscript.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.