Article Text
Abstract
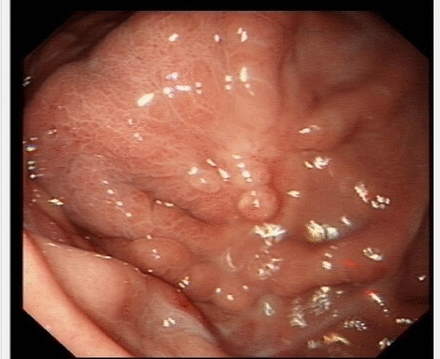
Introduction A 65-year-old woman with type 3 intestinal failure secondary to scleroderma of the gut (limited cutaneous sclerosis (centromere positive) and rheumatoid arthritis (anti-cyclic citrullinated peptide (CCP) and rheumatoid factor positive)) on home parenteral nutrition since 2011 underwent a venting PEG replacement in 2015 for intractable vomiting due to gut dysmotility and small bowel bacterial overgrowth, poorly responding to cyclical antibiotics. An endoscopy was undertaken for planned PEG review for consideration of elective replacement (figure 1).
Initial endoscopy.
Based on this endoscopy, her case was discussed at a multidisciplinary team meeting and the anaesthetic risk of laparotomy to remove the PEG was deemed too high (previous endoscopic PEG exchange under sedation had been poorly tolerated due to tube removal through the oesophagus (possibly affected by scleroderma), and necessitated anaesthesia). Therefore, it was decided to insert a new venting PEG endoscopically alongside the previous buried PEG (cut short and clamped) with the plan to remove the old one at a later date.
Questions
What is shown during the initial endoscopy?
What is shown during follow-up endoscopy?
- nutrition
- nutritional support
- endoscopic gastrostomy
- artificial nutrition support
- enteral/parenteral nutrition
Statistics from Altmetric.com
Footnotes
Contributors NS drafted the first version of the article, and RC and SCC provided the case and revised the first version. NS, RC and SCC revised the second version and agreed for final submission.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests NS has received educational training from Tillots Pharma. SCC has received honoraria from Baxter and Novartis and educational sponsorship from Takeda and Fresenius-Kabi/Calea.
Provenance and peer review Not commissioned; externally peer reviewed.